- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0058 | Human TRPV3 Stable Cell Line-HEK293 | TRPV3 | Human | Epithelial | INQUIRY |
Transient receptor potential (TRP) channels are non-selective cation channels widely distributed in a variety of tissues and organs. According to amino acid sequence homology, 28 types of mammalian TRP channels can be divided into TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPP (Poly cystin), TRPA (Ankyrin), TRPML (Mucolipin) and TRPN (NOMP-C) seven sub-families. TRP channels are involved in a variety of physiological processes in the body, such as mechanical perception, temperature perception, cell proliferation and differentiation, and cellular immunity. The TRPV channel family is currently the most researched TRP channel. In particular, the TRPV1 channel has been proven to play an important role in pain perception.
The TRPV3 channel is a channel of the same family as TRPV1, and its amino acid sequence has about 40% homology with the TRPV1 channel, so the two have similar characteristics in some respects. However, TRPV3 channels also have their own unique aspects in terms of distribution and channel characteristics. For example, TRPV1 channels are mainly distributed in sensory neurons, while TRPV3 channels are mainly distributed in skin keratinocytes. TRPV1 channel does not have sensitization characteristics, while TRPV3 channel has sensitization characteristics.
The Gene Structure and Expression of TRPV3
In 2002, three scientific research groups such as Peier, Xu and Smith reported almost simultaneously on the cloning and functional identification of the TRPV3 gene. In humans, the Trpv3 gene is located on chromosome 17p13, is about 47.5 Kbs in length, contains 18 exons, and is next to the Trpv1 gene (the spacing is about 7.45 Kbs). The mouse Trpv3 gene is located on chromosome 11B4, with a total length of about 30 Kbs, containing 18 exons, and is also close to the Trpv1 gene (spacing about 7 Kbs). Due to alternative splicing, the TRPV3 channel protein encoded by the human Trpv3 gene has several different spliceosome forms (such as 790 aa, 791 aa and 765aa), usually in the form of 790 aa. TRPV3 is a non-selective cation channel with high selective permeability to Ca2+ (PCa2+/PNa+ value is about 10). The pore area of the channel will also change with the intensity of stimulation, such as when subjected to specific excitement. When stimulated by the drug, the pore area will expand. In addition, TRPV3 channel current has obvious outward rectification characteristics, and its single channel conductance value is about 170 pS.
At present, Cao et al. have successfully analyzed the structure of TRPV1 channel protein using single-particle cryo-electron microscopy technology, but the structure of TRPV3 channel protein has not yet been resolved. Similar to the structure of the TRPV1 channel protein, it is speculated that the TRPV3 monomer also has a six-pass transmembrane structure, and its N-terminal and C-terminal ends are both in the cell. The recessed ring between S5 and S6 forms a pore region for ions pass. There are 6 ankyrin repeats at amino acid residues 167 to 363 at the N-terminus.
TRPV3 channels are mainly distributed in skin keratinocytes and cells around hair follicles. The distribution positions of different species are somewhat different. TRPV3 channels are expressed in human sensory nerve fibers (such as dorsal root ganglia and trigeminal ganglia) and the central nervous system. In rodent sensory ganglia, the expression of Tr pv3 was only detected at the transcription level, and the expression of TRPV3 channels was not detected at the protein level. In addition, the expression of TRPV3 channels has also been found in other parts of humans and rodents, such as the mouth, nostrils, trachea, and the end of the colon. Recently, the expression of TRPV3 channels has also been detected in mouse adipocytes and radial uterine artery.
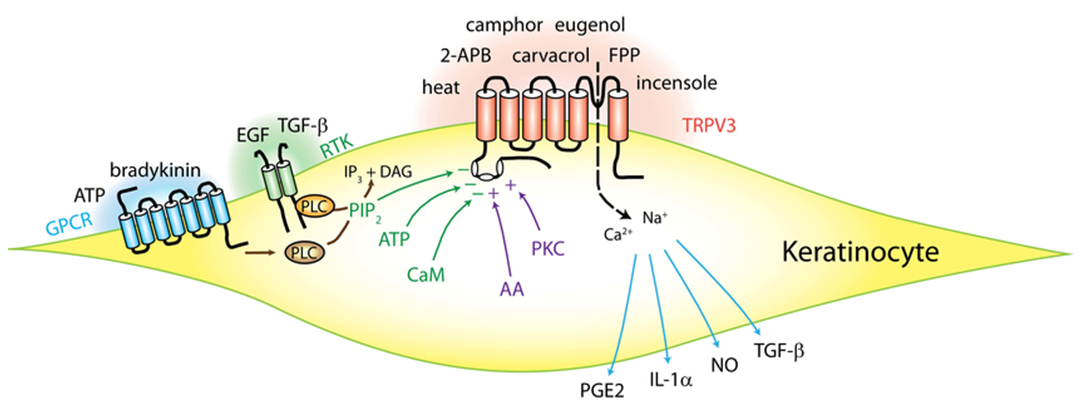
Figure 1. Schematics of activation, regulation and functions of transient receptor potential vanilloid subtype 3 (TRPV3) in keratinocytes. (Huang SM, et al.; 2013)
TRPV3 is a non-selective cation channel that is mainly activated by heat (32 ~ 39 ℃). Studies have shown that, compared with wild-type mice, mice that have knocked out the TRPV3 gene show impaired selection of the optimal temperature, but respond normally to other sensory models. Studies on CHO cells transfected with TRPV3 show that capsaicin, low pH (pH=5.4), and low osmotic pressure cannot activate TRPV3. Only when the temperature rises to 33 ℃, the non-selective cation channel starts to be activated. It leads to Ca2+ internal flow, and the current increases with increasing temperature within a certain range. After repeated thermal stimulation, TRPV3 is sensitized to thermal activation. For example, when the stimulation temperature rises from room temperature to 45 ℃, if only one temperature stimulation is given, the current of TRPV3 is very small. If the temperature change stimulation is repeated, TRPV3 current response increased significantly, and this phenomenon was also observed in TRPV1 and TRPV2. Given the TRPV channel non-competitive blocker ruthenium red, TRPV3 thermally induced current was significantly reduced; while given TRPV1's competitive blocker capsaicin, TRPV3 thermally induced current did not weaken. Therefore, it is believed that TRPV3 is a non-selective cation channel activated by thermal stimulation.
References
- Xu H, et al.; TRPV3 is a calcium-per-meable temperature sensitive cation channel. Nature. 2002, 418 (6894): 181-186.
- Moqrich A, et al.; Impaired thermosen-sation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005, 307 (5714): 1468-1472.
- Peier AM, et al.; A heat sensitive TRP channel expressed in keratinocytes. Science. 2002, 296 (9): 2046-2049.
- Lee Y, et al.; Transient receptor potential vanilloid type 1 antagonists: a patent review (2011-2014). Expert opinion on therapeutic patents. 2015, 25(3): 291-318.
- Xu H, et al.; TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002, 418 (6894):181-186.
- Peier AM, et al.; A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002, 296 (5575):2046-2049.
- Smith GD, et al.; TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002,418(6894): 186-190.
- Liao M, et al.; Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature, 2013, 504(7478):107-112.
- Huang SM, et al.; Targeting TRPV3 for the Development of Novel Analgesics. Open Pain J. 2013;6(Spec Iss 1):119-126.
Inquiry
