- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- trpv4
trpv4
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0203 | Human TRPV4 Stable Cell Line-CHO | TRPV4 | Human | Epithelial-like | INQUIRY |
Transient receptor potential channels (TRP channels) are a family of voltage-independent cation channels originally cloned from Drosophila, including seven subfamilies, namely TRPV, TRPM, TRPC, TRPP, TRPML, TRPA, and TRPN. The TRPV subfamily is further divided into six members, TRPV1-6. Among them, TRPV4 is widely distributed in the body, participates in the pathophysiological mechanism of various diseases, and plays an important role in the occurrence and development of diseases.
Structure and Physiological Function of TRPV4
TRPV4 is a tetrameric structure, similar to voltage-gated potassium channel (KV). The channel protein consists of 871 amino acids with 6 transmembrane domains (TM1-6) per unit. There is an ion channel pore between TM5 and TM6 that allows the passage of various cations such as calcium ions. At the N-terminal tail, there is a very short TRPV4 phosphoinositide-binding site (PIBS), which can sense various physical and chemical stimuli (such as arachidonic acid metabolites and dacosatrienoic acid) activates TRPV4. There is another proline-rich domain (PRD) at this end, which may be involved in the physical activation of TRPV4. The C-terminal tail contains a calmodulin binding domain (CaM), which is the site of protein interaction. Current studies have shown that TRPV4 is expressed in both endothelial cells and vascular smooth muscle cells in the vasculature, and is involved in the regulation of vascular tone. However, the function of TRPV4 is not limited to this. A large number of studies have shown that Trpv4 knockout mice have obvious lesions, such as hearing, temperature, and pain receptor impairment, and are more prone to obesity. In rat dorsal root ganglia, Trpv4 channels are co-expressed with substance P and calcitonin gene related peptide (CGRP), and activation of Trpv4 promotes the release of Cgrp and substance P, while inducing mechanical hyperalgesia, so inhibition of TRPV4 can prevent the release of TRPV4-dependent nociceptive peptides and reduce pain. In the central nervous system, TRPV4 is a key regulator of neuronal excitation in the hippocampus and is involved in maintaining astrocyte homeostasis. In addition to the above physiological functions, TRPV4 is also involved in the pathophysiological process of various diseases.
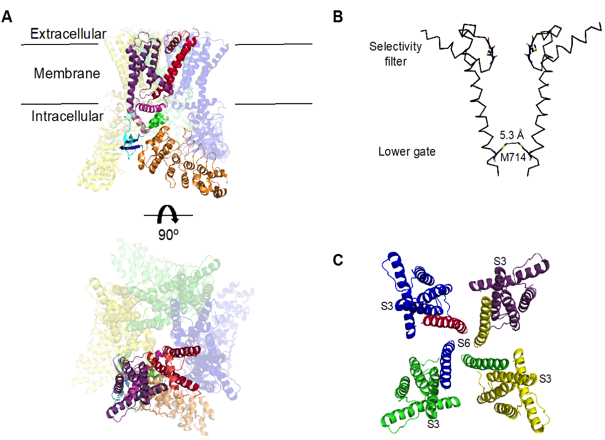
Figure 1. Structure of the TRPV4 channel. (Tamara Rosenbaum, et al.; 2020)
TRPV4 and Diseases
TRPV4 and Cardiovascular System
TRPV4 and cardiovascular system TRPV4 is widely present in the cardiovascular system, mainly expressed in vascular endothelial cells, vascular smooth muscle cells and fibroblasts, etc. It can sense hemodynamic changes and participate in the maintenance of cardiac homeostasis.
I/R Injury and Congestive Heart Failure
During myocardial ischemia-reperfusion (I/R), TRPV4 can be activated and participate in the occurrence of I/R. Studies have found that in the mouse I/R model, the use of Trpv4 inhibitor HC-067047 can significantly reduce the size of myocardial infarction and improve cardiac function in mice, while the use of Trpv4 agonists has the opposite effect. The mechanism of its involvement in I/R injury may be that activation of Trpv4 channels induces calcium influx, and elevated calcium ions promote the release of reactive oxygen species, which then induces depolarization of mitochondrial membrane potential and opens mitochondrial permeability transition pore (MPTP), eventually leading to myocardial damage.
Hypertension
Previous data have shown that under normal physiological conditions, TRPV4 mainly mediates vasodilatory responses in endothelial cells. On the one hand, when the TRPV4 channel in endothelial cells is activated, calcium ion influx induces the production of vasodilator NO, which causes vasodilation; The activation of intermediate conductance potassium channel/small conductance potassium channel (IKCa/SKCa) and the activation of large conductance potassium channel (BKCa) on vascular smooth muscle, the increased intracellular calcium ions reversely promote the generation of reactive oxygen species ( reactive oxygen species, ROS) and form H2O2, which can induce depolarization of vascular smooth muscle, leading to vasodilation. Previous studies have found that in the aorta of hypertensive mice, acetylcholine (Ach) can induce vascular endothelium-dependent contraction, and this contraction response is inhibited under the premise of knocking out TRPV4 or using an inhibitor, indicating that inhibition TRPV4 can antagonize Ach-dependent vasoconstriction, which may involve the TRPV4/cPLA2/COX-2/PGF2a signaling pathway.
TRPV4 and Lung Disease
Many ion channels are involved in the pathogenesis of lung diseases including asthma, chronic obstructive pulmonary disease (COPD), pulmonary hypertension, acute lung injury (ALI), and idiopathic fibrosis (IPF). Recent studies have shown that TRPV4 is closely associated with respiratory diseases, including ALI, IPF, and pulmonary hypertension.
TRPV4 and Bone Diseases
TRPV4 is a major ion channel on the chondrocyte membrane that regulates osmotic pressure, and its absence may contribute to the progression of osteoarthritis. In porcine chondrocytes, TRPV4-mediated calcium signaling modulates osmotic pressure-induced volume changes and releases prostaglandin E2. IL-1β-treated cells also showed swelling due to hypoosmolarity compared to cells not treated with interleukin-1β (IL-1β). However, swelling subsided with the addition of a TRPV4 activator. This suggests that TRPV4 may be involved in the inflammatory response induced by hypotonicity. In nucleus pulposus cells, hypotonicity also activates TRPV4 channels, promotes calcium signaling pathway opening and enhances gene expression of proinflammatory cytokines. Some researchers have proposed that reducing the molecular osmotic pressure between tissues after proteoglycan degradation can increase TRPV4 signaling, enhance the production of pro-inflammatory cytokines, and promote matrix breakdown. The mechanism of hypotonic activation of TRPV4 in chondrocytes includes the phosphorylation of extracellular signal-regulated kinase ERK1/2 and the expression of the osmotic pressure-related hyperosmolarity-inducing factor NFAT5, but the mechanism of NFAT5 in chondrocyte TRPV4 remains unclear. Overexpression of TRPV4 in chondrocytes may lead to calcium overload or amplification of unfavorable signaling pathways, but low expression also disrupts extracellular matrix homeostasis. So activating chondrocyte TRPV4 may have a dual effect. Data from the current study indicate that the outcome of activation of TRPV4 depends on the state of the extracellular matrix. Therefore, partial inhibition of TRPV4 transduction pathway rather than complete knockout or complete inhibition would be a new direction to restore chondrocyte and disc extracellular matrix function.
TRPV4 and Digestive System
Inflammatory bowel disease (BID), including Crohn's disease, ulcerative colitis and indeterminate colitis, seriously affects the quality of life of patients. The scientists confirmed the expression of TRPV4 in human colonic epithelial cells by quantitatively detecting TRPV4 channel mRNA levels in colon tissue samples (Caco-2 and T84 lines) from BID patients and healthy controls, but there was no significant difference in expression between the two. However, some studies have found that compared with non-BID patients, the expression of TRPV4 mRNA in colonic epithelial cells of BID patients is up-regulated. Activation of Trpv4 in colonic epithelial cells promotes the release of inflammatory cytokines and induces intestinal inflammation in normal mice. At the same time, under inflammatory conditions, the expression of Trpv4 was significantly increased in small intestinal epithelial cells and colonic epithelial cells. In addition, central nervous cell Trpv4 is also involved in visceral hypersensitivity and hyperalgesia in mice and BID patients. These results suggest that TRPV4 may play a role in intestinal inflammation, and provide new ideas for clinical treatment of BID.
TRPV4 and Tumors
TRPV4 affects the proliferation, differentiation, migration and apoptosis of tumor cells by regulating calcium ions and producing isoforms, and plays an important role in the occurrence and development of tumors. Studies have shown that activation of TRPV4 can stimulate the proliferation of hepatic stellate cells and indirectly induce the occurrence of liver cancer. The study found that TRPV4 may play a negative regulatory role in the abnormal proliferation of cervical cancer Hela cells. Activation of calcium-sensing receptors (CaSR) can stimulate the proliferation, differentiation, migration and apoptosis of gastric cancer cells, but not normal cells. Studies have shown that in human primary gastric cancer, the expression of CaSR is enhanced, and the increased CaSR activates the TRPV4 channel to promote the influx of calcium ions, and further activates phosphatidylinositol 3-kinase (PI3K)/AKT/β-catenin and MPKA signaling pathway, thereby promoting the proliferation of tumor cells. However, the specific mechanism of CaSR activation of TRPV4 is still unclear, and it is certain that it is related to the phospholipase C activity of TRPV4. Recent studies have found that TRPV4 is also expressed in human melanoma cells. Following administration of the TRPV4 activator GSK1016790A to a melanoma cell line (A375), rapid cellular disorganization, nuclear densification, and cell shedding were observed in most A375 cells. This indicates that pharmacological activation of TRPV4 can lead to disorder, apoptosis and necrosis of human melanoma cells, revealing that TRPV4 agonists may be used as a new substitute for clinical adjuvant treatment of human melanoma.
References
- Voets T, et al.; Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002, 277(37): 33704-33710
- Garcia EA, et al.; Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc Natl Acad Sci USA. 2013, 110(23): 9553-9558.
- Sousa VJ, et al.; Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br J Pharmacol. 2014, 171(10): 2508-2527.
- Zhang P, et al.; TRPV4 (transient receptor potential vanilloid 4) mediates endothelium-dependent contractions in the aortas of hypertensive mice. Hypertension. 2018, 71(1): 134-142.
- Michalick L, et al.; Transient receptor potential vanilloid 4 and serum glucocorticoid-regulated kinase 1 are critical mediators of lung injury in overventilated mice in vivo. Anesthesiology. 2017, 126(2): 300-311
- Balakrishna S, et al.; TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014, 307(2): L158-L172
- Tamara Rosenbaum, et al.; TRPV4: A Physio and Pathophysiologically Significant Ion Channel. Int. J. Mol. Sci. 2020, 21(11), 3837.
Inquiry
