- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- TRPV6
TRPV6
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0204 | Human TRPV6 Stable Cell Line-HEK293 | TRPV6 | Human | Epithelial | INQUIRY |
The V family of transient receptor potential (TRPs) proteins includes 6 channel proteins, of which TRPV1~4 are mainly related to sensations such as heat, acid, stretching, and exogenous chemical stimulation, while TRPV5 and TRPV6 are highly selective for Ca2+. TRPV5 is mainly expressed in the mammalian kidney, and TRPV6 has a broader expression spectrum. The two calcium channels are closely related to the regulation of calcium homeostasis. The TRPV6 gene is located on the long arm of human chromosome 7 and contains 15 exons with a length of about 15.7 kb. The N-terminus of the full-length endogenous human TRPV6 protein is 40 amino acid residues longer than the theoretically translated protein. This is because the translation of TRPV6 starts from the ACG upstream of the AUG, and decodes the corresponding threonine into methylthio amino acid. Translation from non-AUG codons in the TRPV family is unique to TRPV6. The TRPV6 channel consists of four identical subunits, each with six transmembrane segments, forming an inward rectifying Ca2+-selective ion channel. TRPV6 is mainly distributed in the small intestine, epididymal epithelium, placenta, prostate and exocrine pancreas. It can promote the absorption of Ca2+ in the small intestine. In the epididymis, TRPV6 maintains semen at a low calcium level and protects sperm from being killed.
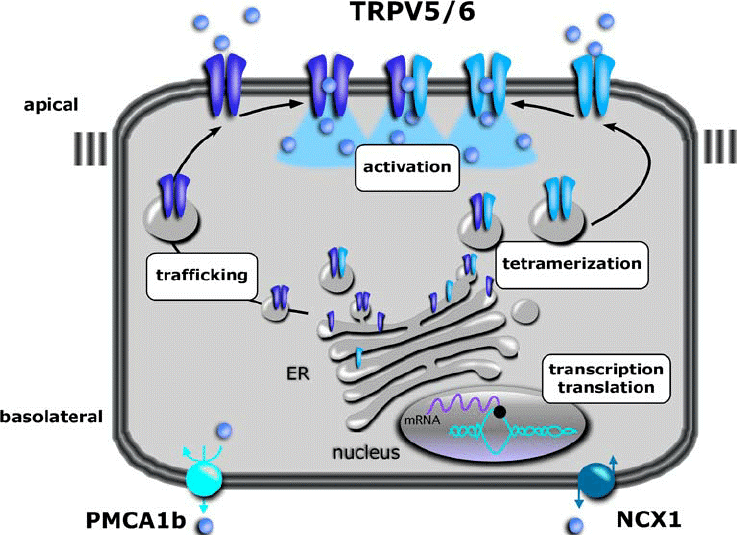
Figure 1. Overview of levels at which TRPV5 and TRPV6 are regulated. (Nijenhuis T, et al.; 2005)
Discovery of TRPV6
Peng et al. (2000) used rat Cat1 as a probe, screened the small intestine cDNA library, and then performed PCR analysis to isolate the cDNA encoding TRPV6, which they called Cat1. Sequence analysis showed that the TRPV6 protein has 725 amino acids and is about 90% similar to the rodent homologous protein. The cytoplasmic N-terminal of TRPV6 has 3 ankyrin repeats, multiple phosphorylation sites, and 6 extracellular pores region, the transmembrane segment of the N-glycosylation site, and the intracellular C-terminus. Northern blot analysis showed that TRPV6 transcripts of 3.0 kb and 10.5 kb were expressed in placental, pancreatic, prostate and colorectal cancer cell lines, with lower expression in kidney and small intestine. Among them, the 3.0 kb transcript was more abundant. The 2.2 kb transcript is expressed in the testis. In the gastrointestinal tract, TRPV6 is expressed in the duodenum, in the esophagus, stomach and jejunum, but not in the ileum.
RPV6 and Disease
Neonatal Skeletal Abnormalities
Ca2+ not only needs to maintain the basic functions of cells during the embryonic stage, but also participates in the formation and mineralization of bones. Therefore, the blood calcium concentration of the newborn needs to be higher than that of the mother. The formation of this high blood calcium depends on the active transmembrane transport of Ca2+. If the transporting function of Ca2+ is decreased or absent, the fetus will cause skeletal hypoplasia due to lack of Ca2+. Animal experiments have shown that Trpv6 knockout mice have the characteristics of small embryos, light body weight, short femurs, and less calcification, and there are still cortical bone microstructure damage in adult mice.
Bone and Cartilage Disorders
TRPV6 exists in both osteoblasts and osteoclasts, and plays a role in calcium transport, regulating the dynamic balance of bone formation and bone resorption. The researchers found that the bone microstructure of Trpv6 knockout mice was significantly damaged, and the number and surface area of osteoclasts were significantly increased, indicating that TRPV6 may be a key regulatory factor in the process of osteoclast differentiation and bone resorption. Further studies found that TRPV6 negatively regulates osteoclasts through the IGF-PI3K-AKT pathway, and knockdown of Trpv6 reduces its inhibitory effect on osteoclasts.
TRPV6 also plays a role in articular cartilage and may be involved in the development of osteoarthritis. The researchers found that the expression of TRPV6 in the articular cartilage of patients with osteoarthritis was significantly decreased, and Trpv6 knockout mice developed osteoarthritis earlier than wild-type mice, and the deletion of Trpv6 reduced the secretion of extracellular matrix and slowed down cartilage cell proliferation and increased chondrocyte apoptosis, leading to osteoarthritis.
Kidney Stones and Hypercalciuria
Hypercalciuria, the most common metabolic abnormality leading to nephrolithiasis, is associated with genetic factors, and its pathogenesis is due to increased intestinal Ca2+ absorption and/or decreased renal tubular Ca2+ reabsorption. The study showed that the urinary osmotic pressure of Trpv6 knockout mice was reduced, and the urinary Ca2+ excretion was significantly increased. Scientists carried out TRPV6 gene detection and functional analysis on 170 patients with renal calcium stones, and found that the gain-of-function TRPV6 mutation increased the risk of renal calcium stones, demonstrated that TRPV6 is involved in the disease process of nephrolithiasis and hypercalciuria.
Inflammatory Bowel Disease and Chronic Pancreatitis
In inflammatory bowel disease, the expression level of TRPV6 in the colon tissue of patients with active ulcerative colitis was significantly higher than that of the control group, which may be involved in the pathological process of ulcerative colitis. Crohn's disease patients are often accompanied by decreased bone mineral density. Researchers found that the expression of TRPV6 in the duodenum was down-regulated in a mouse model of Crohn's disease. Similar results were observed in humans.
TRPV6 and Tumor
TRPV6 is closely related to the occurrence and progression of various tumors, and its overexpression is associated with increased tumor invasiveness. It is worth noting that the expression of TRPV6 is much higher in ovarian, prostate and pancreatic cancers than in normal tissues. In prostate tumors, TRPV6 positivity indicates that the tumor has the ability to invade extraprostatic tissues and metastasize out of the prostate capsule, indicating a poor prognosis. Breast cancer biopsies have shown that TRPV6 mRNA expression is 2- to 15-fold higher than in normal breast tissue, and increased TRPV6 expression is associated with poor prognosis in estrogen receptor-negative breast cancer patients. TRPV6 is also directly related to the development and prognosis of pancreatic cancer, and the survival rate of patients with increased expression is reduced; in vitro experiments have shown that silencing TRPV6 in pancreatic cancer cell lines can reduce the proliferation and invasion ability of cancer cells, and initiate cell apoptosis. lead to cell cycle arrest. In addition, the expression of TRPV6 in colon cancer, ovarian cancer and thyroid cancer was higher than that in normal tissues.
References
- Peng, J.-B., et al.; Human calcium transport protein CaT1. Biochem. Biophys. Res. Commun. 2000, 278: 326-332.
- Peng, J.-B. TRPV5 and TRPV6 in transcellular Ca (2+) transport: regulation, gene duplication, and polymorphisms in African populations. Adv. Exp. Med. Biol. 2011, 704: 239-275.
- Stewart J M. TRPV6 as a target for cancer therapy. J Cancer. 2020, 11(2): 374-387.
- Burren C P, et al.; TRPV6 compound heterozygous variants result in impaired placental calcium transport and severe undermineralization and dysplasia of the fetal skeleton. Am J Med Genet A. 2018, 176(9): 1950-1955.
- Bianco S D, et al.; Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res. 2007, 22(2): 274-285.
- Huybers S, et al.; Murine TNF (DeltaARE) Crohn's disease model displays diminished expression of intestinal Ca2+ transporters. Inflamm Bowel Dis. 2008, 14(6): 803-811.
- Nijenhuis T, et al.; TRPV5 and TRPV6 in Ca(2+) (re)absorption: regulating Ca(2+) entry at the gate. Pflugers Arch. 2005, 451(1):181-92.
Inquiry
