- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
The Patch-Clamp Technique
Cellular physiological properties are closely related to the biological, chemical and physical properties of cells. The patch clamp technique is a laboratory technique in electrophysiology, which is used to study ionic currents in individual isolated living cells, tissue sections, or patches of cell membrane, investigating cellular physiological functions. The development of the patch-clamp technique has given electrophysiologists new prospects. It allows high-resolution current recordings not only of whole cells, but also of excised cellular patches.
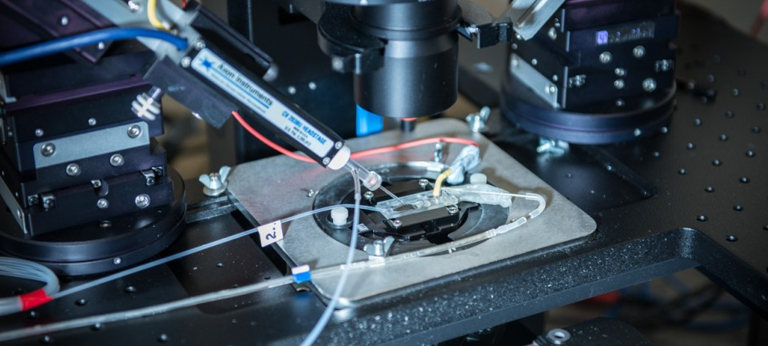
Requirements of Laboratory Work:
- Highly sensitive equipment
- Experimenter with physical and biological background
Principles of Patch Clamp Recording
The number of positive and negatively charged ions inside a neuron differs from number found on the outside. This imbalance produces a voltage difference, or membrane potential, of about -70 mV, meaning that the inside is more negative than the outside. Ion channels help maintain the gradient by controlling the movement of ions across the cell membrane, which are essentially electrical currents.
The patch clamp rig includes a glass micropipette, which contains both an ionic solution and a chlorinated silver electrode for measuring voltages and currents. The tip of the micropipette has a polished, one micron opening that encloses a small area of membrane. To eliminate background noise from ions within the bath solution, a high-resistance seal is formed between the pipette and membrane patch. Because the resistance of the seal is in the gigaohm range, it is known as a gigaohm seal. The electrode within the pipette is connected to an amplifier that can amplifies current and voltage fluctuations that are the result of the movement of ions through channels in the plasma membrane. With the amplifier, researchers can clamp or artificially set the membrane potential at specific voltages. The amplifier regulates how much current must be added through the silver electrode in order to keep the voltage constant. Since different voltage-gated ion channels open at specific voltages, opening events are represented by the variations in the profiles of the measured currents. Alternatively, scientists can force a specific current through the electrode and record the resulting changes in potential. In this “current clamp” configuration action potentials can be recorded.
Patch Clamp Configurations
- Cell-attached configuration. The micropipette is simply sealed to the membrane of an intact cell, and a tight seal is formed by suction with the periphery of the micropipette orifice. Suction is normally released once the seal has formed, but all micropipette current has been eliminated except that flowing across the delineated membrane patch.
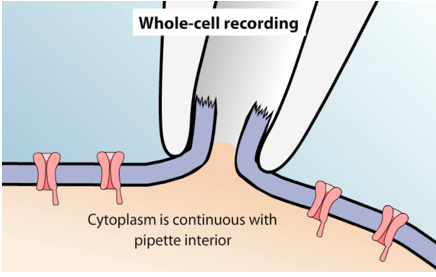
- Whole-cell configuration. The membrane within the micropipette is ruptured with a brief pulse of suction to provide access to the cell’s interior. The gigaseal is maintained, hence it excludes leakage currents that happen in the above configuration. Then the microelectrode measures the current due to the ion channels of the whole cell, which is very similar to a conventional microelectrode penetration.
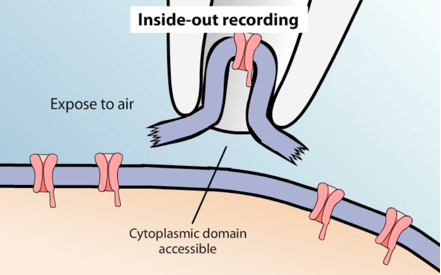
- Inside-out patch. The micropipette first forms a seal with the cell, then is pulled back quickly, ripping a piece of the membrane off and exposing the inside surface to the bath solution. Inside‐out patches can be obtained by membrane broken outside the pipette with a short exposure to air or withdrawal from Ca‐free medium. This allows for the cytoplasmic side of the channels to be exposed to different chemicals applied to the bath. It can be used to investigate the cytoplasmic regulation of ion channels.
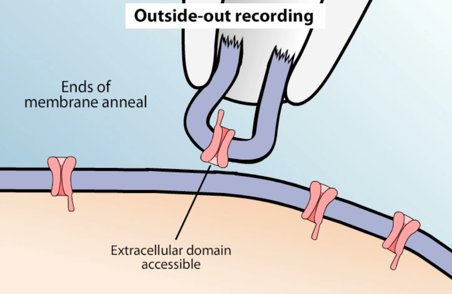
- Outside-out patch. After the cell membrane is ruptured with a pulse of suction, a cytoplasmic bridge becomes more and more narrow as the separation between pipette and cell increases during withdrawal, until a piece of membrane forms a convex seal across the tip of micropipette. In this setup, the extracellular face of the channel can be exposed to experimental treatments. It may be used to investigate the behavior of single ion channels activated by extracellular receptors.
- Perforated patch configuration. Here, chemicals such as antibiotics are added to the micropipette to make small holes in the membrane providing access to the cytosol.
Applications in Neuroscience
Patch clamp recording is an extremely useful technique for investigating the biophysical properties of the ion channels that control neuronal activation, as well as for screening drugs in pharmaceutical industry.
- Biophysics Study
Whole-cell patch clamp is a valuable tool for measuring the response of single cells to stimulation. Furthermore, paired recordings can be used to investigate the impact of neuron firing on excitable target cells, like muscle. For example, whole-cell patch clamp can be used to stimulate firing of a motor neuron, while recordings are simultaneously taken from the muscle fiber it controls. Clear relationships between neuronal excitation and muscle activity are observed. With its exquisite temporal sensitivity to changes in voltage and currents, patch clamp recording will continue to be instrumental in understanding the biophysics of channels and neurons.
- Drug Screening
Channels can be exposed to experimental molecules to study their impact on channel activity and cellular function. Ion channels make excellent drug targets. When test compounds are added to the micropipette or bath solutions, patch clamp recordings can be used to directly test the effect of drugs, like nicotine, on neural activity. The principle of applying negative pressure to form a high resistance seal has even been applied to construct high throughput devices, which can record from numerous cells simultaneously for drug screening applications.
Related Section
Inquiry

