- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0062 | Human ACCN2 Stable Cell Line-CHO | ASIC1 | Human | Epithelial-like | INQUIRY |
pH is an important physiological parameter and an important indicator of homeostasis. Under normal physiological environment, pH is adjusted very precisely to achieve internal environment stability. Based on the unique position of hydrogen ions in the body, scientists believe in the hypothesis: since the stability of pH is so important, there must be a unique receptor substance in the body, which can sense tiny changes in the concentration of hydrogen ions, and this unique sensory substance should be widely distributed in the body. In 1980, Krishtal et al. first recorded a current on nerve cell membranes that can be activated by hydrogen ions, and believed that there may be proton receptors on the cell membrane. For a long time to come, the research on this phenomenon did not make much progress. In 1997, Waldmann et al. cloned acid-activated channel proteins for the first time and named them acid-sensitive ion channels (ASICs). Because of their outstanding work, the research on ASICs has entered a new era.
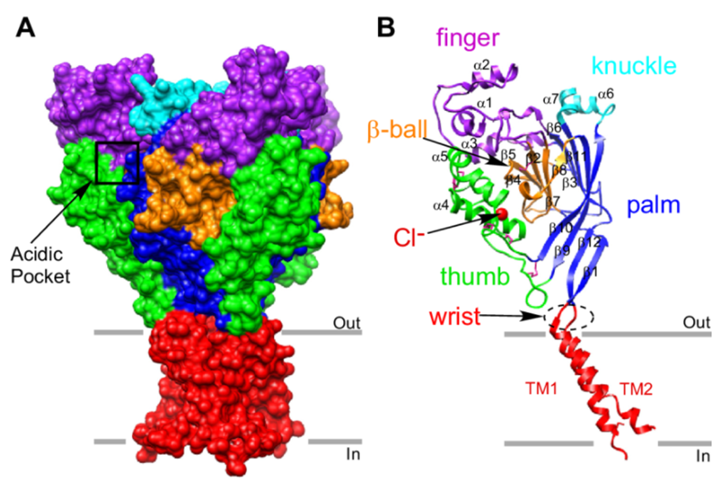
Figure1. Overview of the acid-sensing ion channel 1 (ASIC1) crystal structure.
ASICs belong to the degenerative protein/epithelial cell Na+ channel (DEG/ENaC) superfamily, which is a type of cation channel that can be activated by H+. In addition to being widely distributed in the central and peripheral nervous system, ASICs also exist in vascular smooth muscle cells and epithelial cells. There are 6 subtypes in mammals: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4. These subtypes are encoded by 4 genes. Each subunit contains about 500 amino acids, and all types of channels have a height conserved structural regions: extracellular loop rich in cysteine, two hydrophobic transmembrane regions (TM1 and TM2), intracellular N-terminal and C-terminal. These subtypes can form various homopolymer or heteropolymer channels, which play an important role in signal transduction and the maintenance of excitability in cells.
ASIC1 Function
Waldman and others expressed rat Asic in Xenopus oocytes and found that Asic produced an amiloride-sensitive cation channel that was instantly activated by rapid extracellular acidification. The main penetrating ion is Na+, but this channel can also penetrate into Li+, K+, Ca(2+) and H+. Ca(2+) inhibits Na+ penetration, which is characteristic of H(+)-gated ion channels in sensory neurons.
Later, Bassler et al. studied the two subtypes, Asic1a and Asic1b, encoded by the alternately spliced transcript of rat ASIC1. The comparison shows that the N-terminus of the two subtypes is different, and the N-terminus of Asic1b is longer. After being expressed in Xenopus oocytes, both channels generate currents that are rapidly activated by pH, and they show similar channel dynamics. Asic1a shows low Ca(2+) permeability, while Asic1b is impermeable to Ca(2+). The researchers identified a domain before transmembrane zone 1, which controls the permeability of divalent cations.
Wemmie et al. targeted human ASIC1A for overexpression into the mouse brain. The transgenic ASIC1A interacts with endogenous mouse Asic1a and distributes to the synaptosome mouse brain. The transgene expression of ASIC1A also doubled the cation current induced by neuronal acid. Among them, ASIC1A showed prominent expression in the amygdala. Overexpression of ASIC1A enhanced fear conditioning, which is an animal model of acquired anxiety. Therefore, researchers believe that ASIC1A and H(+) gated currents may lead to the development of abnormal fear and anxiety in humans.
In addition, studies have found that ASIC1 can also cause acidosis damage to the brain through a mechanism based on membrane receptors, leading to intracellular Ca(2+) toxicity. It also acts as a post-synaptic proton receptor, affecting intracellular calcium concentration and CAMK2 phosphorylation, thereby affecting the density of dendritic spines.
References
- Waldmann, R., et al.; A protein-gated cation channel involved in acid-sensing. Nature. 1997, 386: 173-177.
- Wemmie, J. A., et al.; Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc. Nat. Acad. Sci. 2004, 101: 3621-3626.
- Bassler, E.-L.,et al.; Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J. Biol. Chem. 2001, 276: 33782-33787.
- Xiong, Z.-G., et al.; Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004, 118: 687-698.
- Zha, X., et al.; Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc. Nat. Acad. Sci. 2006, 103: 16556-16561.
Inquiry
