- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0015 | Human ASIC3 Stable Cell Line-HEK293 | ASIC3 | Human | Epithelial | INQUIRY |
| ACC-RI0065 | Human ACCN3 Stable Cell Line-CHO | ASIC3 | Human | Epithelial-like | INQUIRY |
Acid-sensing ion channel 3 (ASIC3), also known as amilorid-sensitive cation channel 3 (ACCN3) or testis sodium channel 1 (TNaC1), is a protein encoded by the ASIC3 gene. The ASIC3 gene is one of five paralogous genes. The protein they encode forms a trimeric acid-sensing ion channel (ASIC) in mammals. The ASIC gene has splice variants, which encode different proteins called isotypes. These genes are mainly expressed in the central and peripheral nervous system. ASIC can form homotrimers (consisting of three identical subunits) or heterotrimeric channels.
Since Waldmann et al. cloned ASIC in 1997, related research has continued to deepen, and a total of 6 different subunits have been discovered so far: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4. Phylogenetic studies have shown that the 6 subunits of ASIC are all members of the epithelial sodium channel/degeneration factor gene (ENaC/DEG) family. ASIC is an H+ gated, Na+ high permeability cation channel located on the cell membrane. When the extracellular H+ concentration increases, it is activated, and the extracellular Na+ enters the cell through this channel, causing depolarization. At present, the generally accepted model is that the ASIC channel is a polymer of 4 subunits, homopolymers composed of 4 identical subunits or heteropolymers formed by different subunits, and the ion channel is located in the middle of these 4 subunits. Each subunit of ASIC is composed of 500-600 amino acids, and human ASIC3 is composed of 531 amino acids. These amino acids form a long peptide chain, forming two transmembrane regions on the cell membrane: Transmembrane 1 (TM1) and Transmembrane 2 (TM2). The carboxyl (C) and amino (N) ends are both in the cell, and the cell There is a long chain ring outside. There are several conservative areas in this structure that are very important to the function of ASIC. As a receptor, the site that receives H+ stimulation is outside the cell, which is the site that maintains the basic function of the channel. The site related to channel gating is outside the cell close to the glycine (Gly) 430 of TM2; and directly affects the probability of channel opening The sites of, Na+ selectivity and ion permeability are the 9 conservative amino acid sequences close to TM1 in the cell. Through a large number of studies, we have a better understanding of the structure of ASIC3 and found that ASIC3 participates in the formation of different ASIC channels in different organizations. ASIC3 exists as a homopolymer in the rat heart, and ASIC3 expressed in the dorsal root ganglion (DRG) neurons innervating the muscle also forms a homopolymer channel and performs its function.
ASIC3 Distribution
In recent years, people have found that ASIC3 is not only found in the nervous system of mammals such as the brain, trigeminal nerve, gastric nerve, retina, sensory nerve, etc., but also in many other parts such as the heart, lung, bone, skeletal muscle, testis, Taste buds, skin nerve endings, etc. are also found to have ASIC3 expression. Many scholars have reported that there are abundant ASIC3 in DRG neurons. For example, Waldmann et al. applied cloning technology, in situ hybridization technology, and cell electrophysiology to prove the existence, distribution and electrophysiological characteristics of ASIC3 in rat DRG. There are also many discoveries of ASIC3 in other parts of the nervous system.
Electrophysiological Characteristics and Regulation of ASIC3
With the progress of research work, the characteristics and adjustments of ASIC3 subunit channels have become more clear. In comparison, heteromers usually have different pH sensitivity and current characteristics from homopolymer channels, and even ion selectivity also changes, and desensitization is significantly faster than their respective homopolymer channels. These changes are related to functions. The ASIC3 heteromer is more sensitive to acid than the homopolymer channel, its pH range is closer to physiological and pathological changes, and its distribution and function in tissues are more. Moreover, the current mediated by ASIC3 contains two components: rapid inactivation and steady state. These electrophysiological characteristics determine the diversity of its effects. The rapid inactivation part is usually high in amplitude and explosive, which is easy to cause local electrophysiological disorders. If it occurs in the sensory nerve, it will cause hyperalgesia; if it occurs in the heart, it is not only pain, but also may cause arrhythmia. Steady-state components are caused by continuous influx of cations such as Na+, which may not only be related to the nociception of continuous pain, but also cause cell damage and destruction, leading to tissue damage. In addition to H+, many factors such as Gd3+, Zn2+, Ca2+, Mg2+ and other metal ions; neuropeptide SF (NPSF), neuropeptide FF (NPFF), NP-FMRF (Phe-Met-Arg-Phe-NH2), etc. neuropeptides; protein kinases, CIPP (channel-interacting PDZ domain pro-tein), etc., have a regulatory effect on ASIC3 channels.
ASIC3 and Disease
ASIC3 and Myocardial Ischemia
At present, apart from ASIC3 in the heart, no other subunits of ASIC have been reported. When myocardial ischemia is caused by various reasons, acidic substances increase, causing the local H+ concentration of the lesion to increase. The important thing is that ASIC3 is sensitive to pH changes and is involved in angina pectoris induced by myocardial acidification. At the same time, abnormal ASIC3 currents not only cause pain along the sensory nerves, but also may cause the opening of peripheral voltage-gated channels, which may lead to arrhythmia. Benson et al. separated and cultivated the afferent nerves of the heart, and conducted a patch clamp experiment. They found that almost all cardiac sympathetic afferent nerves have a large inward current activated by acidic pH, and the biophysical properties of this current are the same as ASIC3. According to Stephani's research on rats, ASIC3 forms channels in the heart sensory nerve as homopolymers. One particularly important finding is that the channel of ASIC3 can be activated and opened at around pH 7, forming a steep current. In the earliest stage of myocardial ischemia and infarction, the range of pH changes is 6.7-7.1, and ASIC3 is just sensitive and can be activated in this range. The study of Immke et al. further showed that a large amount of ASIC3 exists in the sensory neurons of the heart, and its opening and complete regulation are closely related to the release of lactic acid caused by muscle ischemia.
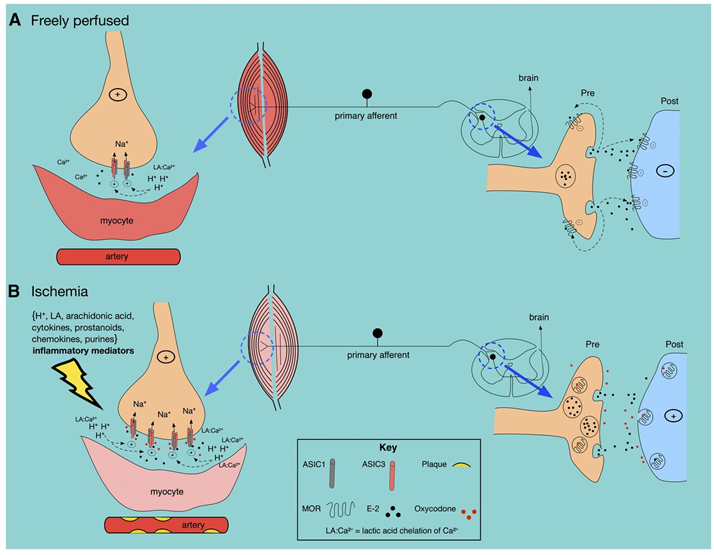
Figure 1. General scheme showing possible opioid- and endomorphin-mediated effects on ASIC channels under freely perfused and ischemic conditions.(Malgorzata Zaremba, et al.; 2019)
ASIC3 and the Perception of Pain, Nociception and Mechanical Stimulation
In recent years, the research on pain has made great progress. Studies have shown that ASIC3 is one of the main acid sensors in the injury and pain caused by acidification conditions. Especially for persistent pain caused by acidosis, ASIC3 may be involved with the catecholamine receptor (TRPV1) to mediate pain and nociception. The heteromeric channel formed by ASIC3 and other subunits is highly sensitive to pH, and the current mediated by ASIC3 contains persistent steady-state components. These electrophysiological characteristics may be closely related to pain sensitivity and persistence. In addition, gene knockout studies also found that ASIC3 may be involved in the regulation of high-intensity pain perception. In a psychological test, Ugawa et al. infused acidic liquid into human skin to cause local pain, and amiloride, a selective blocker of ASIC, can block this pain. Jones et al. applied electroosmosis experiments in volunteers to study acid-induced pain, and found that transdermal electroosmosis caused sustained dose-dependent moderate pain. Subcutaneous injection of amiloride significantly inhibited this pain. The analysis showed that skin acid induced pain, the pain is mainly ASIC3 mediated. Nociceptor ASIC3 and TRPV1 are co-expressed in some DRG neurons, suggesting that the two are jointly involved in pain and persistent pain caused by tissue acidosis. A large number of animal experiments also show that AISC3 is involved in the perception and regulation of acid pain in the skin and muscles.
References
- Kellenberger S, et al.; Epithelial sodium channel/ degenerin fami- ly of ion channels:a variety of functions for a shared structure. Physiol Rev. 2002, 82(3):735-767.
- Babinski K, et al.; Molecular cloning and regional dis- tribution of a human proton receptor subunit with biphasi c functional properties. J Neurochem.1999, 72(1):51-57.
- Sutherland SP, et al.; Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons.Proc Natl Acad Sci USA. 2001,98(2):711-716.
- Sluka KA, et al.; Chronic hyperalgesia in- duced by repeated acid injections in muscle is abolished by the loss of ASIC3 , but not ASIC1. Pain. 2003,106(3):229-239.
- Immke DC, et al.; ASIC3:a lactic acid sensor for cardiac pain. Sci World J. 2001, 1:510-512.
- Ugawa S, et al.; Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human noci ceptors. J Clin Invest. 2002, 110(8):1185-1190.
- Jones NG, et al.; Acid-induced pain and its modulation in humans. J Neurosci. 2004, 24(48):10974-10979.
- Zhang P, et al.; Single channel properties of rat acid-sensitive ion channel-1α, -2a , and -3 expressed in Xenopus oocytes. J Gen Physiol. 2002,120(4):553-566.
- Malgorzata Zaremba, et al.; Opioid-Mediated Modulation of Acid-Sensing Ion Channel Currents in Adult Rat Sensory Neurons. Molecular Pharmacology. 2019, 95 (5) 519-527.
Inquiry
