- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- CACNA2D4
CACNA2D4
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0069 | Human CACNA1A/CACNB3/CACNA2D2 Stable Cell Line-CHO | CACNA2D4 | Human | Epithelial-like | INQUIRY |
CACNA2D4 is a voltage-dependent calcium channel subunit α-2/delta-4. It belongs to the family of α-2/δ subunits and has been extensively studied in the past few decades because of its various functions of calcium channel regulation and its potential as a therapeutic target. Existing studies have shown that CACNA2D4 can change the properties of pore α-1 subunit voltage-gated calcium channels. It consists of two polypeptides, an α-2 subunit and a delta subunit, which are connected by disulfide bonds.
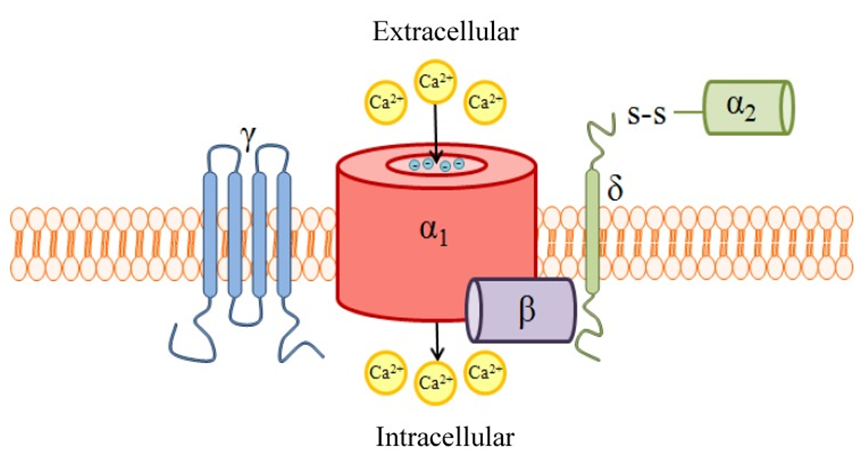
Figure 1. A schematic of high voltage-activated (HVA) voltage-gated Ca2+ channels (VGCC) quaternary structure and subunit composition. (Heidi Hannon, et al.; 2013)
CACNA2D4 is ubiquitously expressed in a variety of endocrine cells, such as paneth cells of the small intestine, erythroblasts of the fetal liver, and reticulum bands of the adrenal glands. At the same time, it can also adjust the calcium current density of calcium channels and the activation/inactivation mechanics of calcium channels.
CACNA2D4 Cloning and Expression
By analyzing the EST of the related sequence of α-2/delta calcium channel subunits, and then sequencing the brain cDNA library by RT-PCR and RACE, Qin et al. determined that the CACNA2D4 gene is about 130kb in length and contains 36 invariant exons (2 ~ 37 exons) and 4 optional exons (11, 1B, 37L and 38 exons). The 37L exon is a continuous extension of the 37 exon and contains an in-frame stop codon.
Later, the researchers cloned CACNA2D4 splice variants with 2 different N-terminals and 2 different C-terminals. One of the variants, CACNA2D4a, encodes a protein of approximately 1120 amino acids with a molecular mass of approximately 126 kD. Further research found that CACNA2D4a has most of the features of the α -2/delta subunit, including the n-terminal signal sequence, the conserved alanine residues between α -2 and the delta polypeptide, a single transmembrane domain, and 6 putative N-glycosylation sites, and 15 conserved cysteines, which combine α -2 and delta peptides to form functional subunits. Among them, CACNA2D4a has 61% amino acid homology with CACNA2D3. Northern blot analysis detected a high level of 7.0 kb CACNA2D4 transcript expressed in the heart and skeletal muscle. Other tissues, including brain, placenta, lung, liver, kidney, and pancreas, have lower expression. Western blot analysis detected CACNA2D4 protein in the pituitary gland and adrenal gland, and its apparent molecular weight was about 160 kD. Lower protein levels are detected in the brain. Immunohistochemical staining detected CACNA2D4 expression in small intestine Paneth cells, fetal liver erythrocytes, adrenal reticular zone cells and pituitary basophils. However, some brain areas such as the cerebellum and cerebral cortex are weakly stained.
CACNA2D4 and Disease
Structural and functional abnormalities of retinal ribbon synapses
The study found that in mice, the expression of CACNA2D4 in the retina of mutant and deletion animals showed a significantly reduced amount of transcription. Further research showed that the cause was caused by a homozygous single nucleotide insertion in exon 25 of Cacna2d4, which resulted in a frame shift and truncated a third of the predicted amino acid sequence. This leads to a lack of normal CACNA2D4 on the retina, which in turn affects ion homeostasis. Since small changes in calcium concentration can regulate a variety of molecular processes, the disorder of calcium homeostasis caused by mutations in Cacna2d4 may disrupt synaptic signal processing in the retina, leading to cone cell failure and early rod cell degeneration. In the study of human retinal diseases, it was also found that nonsense mutations caused CACNA2D4 deficiency. This involves the lack of auxiliary stimulation and the reduction of the normally functioning calcium channels in the patient's retinal synaptic terminals. In addition, the deficiency may lead to an attenuation of the calcium channel density of the synaptic membrane. Abnormal CACNA2D4 protein translation may also cause retinal diseases in patients. If it cannot be effectively degraded, the abnormal CACNA2D4 protein will interact with the corresponding α 1 subunits in the cytoplasmic compartment and reduce their transport to the cell surface, resulting in a decrease in channel density. The reduction of functional CACNA2D4 protein at synaptic ends may lead to inefficient signal transmission from photoreceptors. Therefore, mutations in Cacna2d4 may also affect the corresponding retinal diseases in human patients.
References
- Qin, N., et al.; Molecular cloning and characterization of the human voltage-gated calcium channel alpha-2/delta-4 subunit. Molec. Pharm. 2002, 62: 485-496.
- Wycisk, K. A., et al.; Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest. Ophthal. Vis. Sci. 2006, 47: 3523-3530.
- Wycisk, K. A., et al.; Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am. J. Hum. Genet. 2006, 79: 973-977.
- Heidi Hannon, et al.; Omega-Conotoxins as Experimental Tools and Therapeutics in Pain Management. Marine Drugs. 2013, 11(3):680-99.
Inquiry
