- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0020 | Human CHRNA7 Stable Cell Line-HEK293 | CHRNA7 | Human | Epithelial | INQUIRY |
| ACC-RI0152 | Human CHRNA7 Stable Cell Line-CHO | CHRNA7 | Human | Epithelial-like | INQUIRY |
Neuronal acetylcholine receptor alpha subunit-7, also known as nAChRα7 or CHRNA7, is a protein encoded by the human CHRNA7 gene. The protein encoded by this gene is a subunit of certain nicotinic acetylcholine receptors (nAchR).
nAChRs are members of the superfamily of ligand-gated ion channels that mediate the rapid transmission of signals at synapses. nAChR is considered to be a heteropentamer composed of subunits. The common structure of each subunit is a conserved N-terminal extracellular domain, then three conserved transmembrane domains, a variable cytoplasmic loop, a fourth conserved transmembrane domain, and a short C-terminal cell Outland. The protein encoded by this gene forms ion channels, shows significant permeability to calcium ions, and is the main component of the brain's nicotinic receptors, which are blocked by α-mycotoxins and are highly sensitive to it. Once the receptor binds to acetylcholine, its conformation changes extensively, which affects all subunits and causes ion-conducting channels across the plasma membrane to open. The region where the gene is located has been identified as the main susceptibility locus for juvenile myoclonic epilepsy and a chromosomal location related to the genetic transmission of schizophrenia.
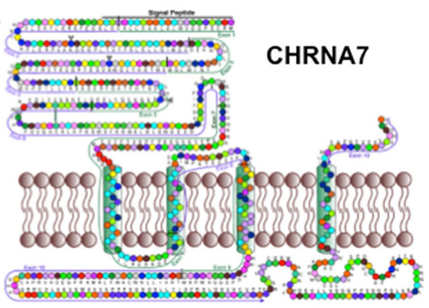
Figure 1. The normal α7 subunit with three glycosylation sites and signal peptide. (Melissa L. Sinkus, M.D, et al.; 2015)
Studies have found that high-affinity nicotinic receptors are usually located in the CNS before synapses, but α7nAchRs have a wider range of localization and functions in both presynapses and postsynapses. They are also expressed in the periphery, including neuroendocrine cells, macrophages and sperm. The Ca2+ permeability of α7nAChRs exceeds that of other nicotinic receptor subtypes and NMDA receptors. Causes cell depolarization to activate voltage-dependent calcium channels (VDCCs) and induce Ca2+ release from the endoplasmic reticulum. The ion selectivity of the receptor seems to be regulated by the transmembrane (TM) TM2 intracellular surface glutamate residues, which are not found in other α subunits. In addition, the normal function of α7nAChRs in mammalian cells requires co-expression Chaperone proteins, such as ric-3 and lynx1, to promote the formation of surface receptors.
CHRNA7 and Disease
Studies have found that the α7 neuron nicotinic receptor gene CHRNA7 on chromosome 15 is widely expressed in the brain and surrounding areas, and plays a variety of important roles in the cognition and immune system. Decreased expression and function of CHRNA7 are related to many diseases, including schizophrenia, bipolar disorder, attention deficit hyperactivity disorder (ADHD), Alzheimer's disease, autism, epilepsy, and learning disabilities.
Schizophrenia
The expression of α7nAChRs decreased in multiple areas of the brain after death in subjects with schizophrenia, including the hippocampus, cortex, and reticularthalamic nucleus. Genetically speaking, the CHRNA7 gene was originally linked to schizophrenia through the dinucleotide marker D15S1360 in intron 2. Linkage is more important for sensory defects P50, which is inherited as an autosomal dominant. It was subsequently found that schizophrenia was positively correlated with other microsatellite markers near 15q13.3 near the CHRNA7 gene, and these markers were not in the repeat region.
Bipolar Disorder
By 125I-α-mycotoxin binding measurement, the expression of α7nAChRs seems to increase in the hippocampus of biphasic patients. The association between the CHRNA7 gene cluster and bipolar disorder has been supported in a number of studies, including genetic linkage, association, and P50 deficiency in neurobiological research. Cognitive deficits in patients with bipolar disorder are similar to those in patients with schizophrenia, involving attention. The sensory processing deficit phenotype (P50 gating) of bipolar disorder patients with psychotic symptoms is also defective. In bipolar schizophrenic affective disorder, P50 deficiency is related to functional mutations grouped in the CHRNA7 proximal promoter.
Autism
The expression of α7nAChR in the brain after death in Rett syndrome and autism is reduced. Like schizophrenia and bipolar disorder, the auditory evoked response is also abnormal. A large-scale genetic linkage study on the autism spectrum showed that the location of the CHRNA7 gene cluster, 15q13.1-q14, has a significant linkage relationship with it. There are also rare chromosomal deletions in autistic patients. These results indicate that common attention and attention deficits are found in schizophrenia, bipolar disorder, and autism. In addition, the CHRNA7 gene locus may have an important role.
Alzheimer's Disease
Alzheimer's disease (AD) is represented by the cholinergic innervation of the cortex, which has the greatest impact on the hippocampus and temporal lobe. Pathological findings include extracellular plaques containing beta amyloid (Aβ) peptides and neurofibrillary tangles with hyperphosphorylated tau protein. It co-localizes with α7nAChR in many regions, including the hippocampus. In the normal brain, soluble Aβ 1-42 exists at a pM concentration, which binds to and activates α7nAChR near the agonist site. However, with the development of AD, the concentration of Aβ 1-42 increased to the nM range, resulting in inactivation of α7nAChRs. Recent reports have shown that the α7 subunit and β2 subunit are assembled together to form α7β2* receptors in selected areas such as the basal forebrain. Their affinity for Aβ 1-42 is enhanced and upregulated by the peptide. The combination of high concentrations of Aβ 1-42 can induce hyperexcitability, which may affect the up-regulation of receptors and the development of AD symptoms.
References
- Melissa L. Sinkus, M.D, et al.; The Human CHRNA7 and CHRFAM7A Genes: A Review of the Genetics, Regulation, and Function. Neuropharmacology. 2015, 96(0 0): 274-288.
- Adams CE, et al.; Development of the alpha 7 nicotinic cholinergic receptor in rat hippocampal formation. Dev Brain Res. 2002; 139:175-187.
- Albuquerque EX, et al.; Mammalian nicotinic acetylcholine receptors: From structure to function. Physiolog Rev. 2009; 89:73-120.
- Ancin I, et al.; Sustained attention as a potential endophenotype for bipolar disorder. Acta Psych Scand. 2010, 122:235–245.
- Allen-Brady K, et al.; . Genome-wide linkage in Utah autism pedigrees. Mol Psych. 2010,15:1006-1015.
Inquiry
