- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0012 | Human HCN2 Stable Cell Line-HEK293 | HCN2 | Human | Epithelial | INQUIRY |
| ACC-RI0100 | Human HCN2 Stable Cell Line-CHO | HCN2 | Human | Epithelial-like | INQUIRY |
The HCN channel is composed of four subunits HCN1-4, which can be assembled in different combinations and conformations. These channels are usually expressed in the brain, heart, and retina. HCN1 is a common isoform in hippocampus, neocortex and cerebellar cortex. HCN2 expression is found in the midbrain and thalamus, while HCN4 is the main subtype in the heart, thalamic nucleus, basal ganglia and olfactory bulb. In addition, when HCN channels are expressed in dendritic cells and cell bodies, they show different subcellular locations and different physiology.
HCN2 Function
HCN channels can cause electrical rhythms in specialized brain neurons and cardiomyocytes. The channel is dual activated by voltage and the combination of cyclic adenosine phosphate (cAMP) and its four cyclic nucleotide binding domains (CNBD). cAMP binds to different concatemers of HCN2 channel subunits, and each channel subunit has a certain number of functional CNBDs. CNBD of each ligand addition are in a manner to promote activation of the channel by overlay.
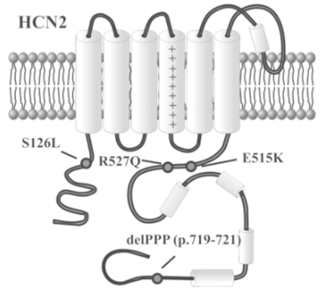
Figure 1. Variants of HCN2 channels associated with epilepsy. (Jacopo C. DiFrancesco, et al.; 2015)
The HCN channel regulates the discharge and excitability of neurons through the hyperpolarized activation current I h (also called h current). The HCN channel is activated by a hyperpolarized state and has constitutive activity at rest. This allows HCN channels to regulate neuronal excitability by stabilizing membrane potentials for excitatory and inhibitory inputs.
HCN2 and Disease
HCN2 and Epilepsy
The genetic loss of HCN channel function obviously leads to epilepsy. Knockout of the HCN2 channel can lead to the phenotype of generalized epilepsy rodents, and inbred rat strains with epilepsy show changes in the function of HCN2 and HCN1 channels. So far, no human hereditary epilepsy lineage is associated with mutations in the HCN channel.
HCN2 and Analgesia
Studies have found that the action potential discharge rate of nociceptors is the main determinant of pain intensity. Possible modulators of action potential excitation include HCN ion channels, which generate inward current I(h) after the membrane is hyperpolarized. Studies have found that deleting the cyclic adenosine monophosphate (cAMP) sensitive component of HCN2 that produces I(h) will hinder the action potential discharge caused by the increase of cAMP in nociceptors. Mice that specifically deleted HCN2 in the nociceptor expressing Na(V)1.8 had a normal pain threshold, but the inflammation did not cause hyperalgesia heat stimulation. After nerve injury, these mice did not respond to thermal or mechanical stimulation. Therefore, neuropathic pain is triggered by the action potential driven by HCN2 in Na(V)1. Another study found that deleting HCN2 subtypes from nociceptive neurons can eliminate heat-induced inflammatory pain and neuropathic pain, but acute pain is not affected. These works indicate that selective HCN2 blockers may have analgesic effects in pain treatment.
HCN2 and Gastrointestinal Function
HCN channels are important regulators of excitability in nerves, heart and other pacemaker cells. In mice, the loss of HCN2 causes cardiac arrhythmia, continuous spike-wave discharge. Recent studies have found that the growth of HCN2 knockout animals is still restricted. To find out the reason, the researchers established mice with complete loss of HCN2 protein, and finally found that homozygous HCN2 protein-deficient mice have abnormal gastrointestinal function, which is characterized by less food consumption and less food consumption than wild-type mice. Delayed gastrointestinal transit. Therefore, the new mutation in HCN2 may cause impaired gastrointestinal motility, thereby causing severe growth restriction in mice with mutations that eliminate HCN2 expression.
References
- Fisher DW, et al.; Loss of HCN2 leads to delayed gastrointestinal motility and reduced energy intake in mice. PLoS One. 2018, 13(2):e0193012.
- Emery EC, et al.; HCN2 ion channels: an emerging role as the pacemakers of pain. Trends Pharmacol Sci. 2012 , 33(8):456-63.
- Sunkara MR, et al.; All four subunits of HCN2 channels contribute to the activation gating in an additive but intricate manner. J Gen Physiol. 2018, 150(9):1261-1271.
- Ming-HuHan, et al.; Neurobiology of Depression. Chapter 12 - Molecular, Cellular, and Circuit Basis of Depression Susceptibility and Resilience. 2019, Pages 123-136.
- Jacopo C. DiFrancesco, et al.; Dysfunctional HCN ion channels in neurological diseases. Frontiers in Cellular Neuroscience. 2015, 6(e1000381):174.
Inquiry
