- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- KCNA10
KCNA10
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0033 | Human KCNA10 Stable Cell Line-CHO | KCNA10 | Human | Epithelial-like | INQUIRY |
KCNA10 encodes a potassium voltage-gated channel and is the tenth member of the shaker-related subfamily, also known as Kcn1 and Kv1.8. This member of the shaker-related subfamily contains six transmembrane domains with repetitions of shaker types in the fourth section. It is specifically regulated by cGMP and is hypothesized to mediate the effects of increased intracellular cGMP. This gene is an intronless gene and is clustered with the genes KCNA2 and KCNA3 on chromosome 1.
KCNA10 Clone
In 1997, Orias et al. cloned the human homologous gene of rabbit KCNA10. Human KCNA10 encodes a putative amino acid protein of approximately 511 amino acids in length, which is 85% identical to the rabbit homologous protein.
Kv1.8 Structure
The structure of Kv1.8 contains six transmembrane domains with a shaker-like repeat and a pore (P) region in the fourth segment. Its most notable feature is the presence of a putative cyclic nucleotide binding (CNB) domain at the COOH terminus. It is specifically regulated by cGMP and is hypothesized to mediate the effects of increased intracellular cGMP-1. A study of this channel revealed that Kv1.8 has an unusual inhibitor. In addition to being inhibited by typical K+ channel blockers, this channel is also sensitive to cyclic nucleotide cation channel inhibitors such as verapamil and pimidine.
Structural comparison revealed that KCNA10 and KCN1 share 90% similarity at the amino acid level, their secondary structures are also the same, and they also have a putative cGMP-binding domain at the COOH end. Although KCNA10 is suspected to have a similar secondary structure to all KCNA channels, its presence at the carboxy terminus of a cyclic nucleotide-binding domain makes it unique among the KCNA family.
KCNA10 Expression
Tissue expression of KCNA10 is a cyclic nucleoside-gated, voltage-activated K channel that can be detected in kidney, heart, and aorta by Northern blot assay. Anti-KCNA10 polyclonal antibodies were prepared and immunocytochemical studies were performed on rat kidneys. The study found that KCNA10 protein was readily detected on the apical membrane of rat proximal tubular cells and also had a weaker signal in glomeruli. Immunocytochemical studies were confirmed by in situ hybridization experiments, which found that KCNA10 was expressed in human proximal tubule cells, glomerular and vascular endothelial cells, and vascular smooth muscle cells. These data suggest that KCNA10 may promote proximal tubular sodium uptake by stabilizing cell membrane voltage.
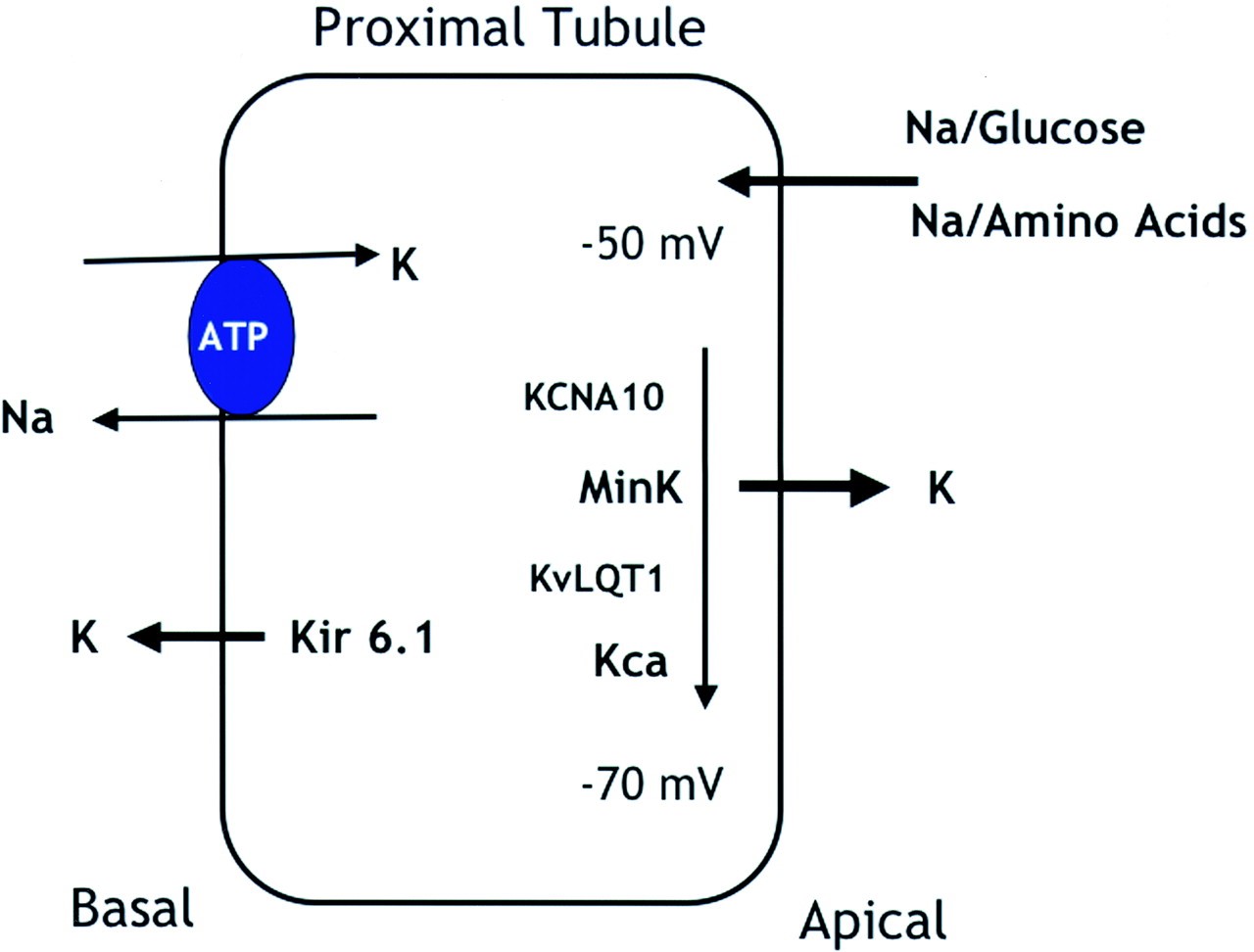
Figure 1. Proposed physiologic role of KCNA10 in the proximal tubule. (Yao X et al.; 2002)
Detection of beta-galactosidase expression in multiple organs of Kcna10TM45 mice using the KCNA10 promoter and enhancer. The brain, heart, lung and liver of both heterozygous and homozygous Kcna10TM45 mice did not show any β-galactosidase expression. Kidney expression could not be assessed due to endogenous β-galactosidase-like activity in wild-type kidneys. The experimental results show that the message expression of KCNA10 is largely similar to that of KCNA4B, mainly in the heart, kidney and skeletal muscle, but not in the brain.
The reporter gene study showed that KCNA10 was mainly expressed in the hair cells of the vestibular organ and the Corti organ of the inner ear, and the hair cells of the inner ear expressed KCNA10 strongly in the hair cells of the Corti organ. The expression of KCNA10 follows a gradient opposite to the pitch gradient. However, KCNA10-null mice exhibited mild hearing impairment and insignificant vestibular dysfunction.
KCNA10 Function
Studies have found that Kv1.8 is involved in renal K metabolism and regulates vascular tone. This plays an important role in the process of blood vessel formation. A recent study showed that a null mutation of KCNA10 in mice causes QT syndrome with marked vestibular and mild hearing impairment. In addition, KCNA10 is also associated with long QT syndrome (LQTS).
References
- Orias, M., et al.; Genomic localization of the human gene for KCNA10, a cGMP-activated K channel. Genomics. 1997, 42: 33-37.
- Yao X et al.; Expression of KCNA10, a voltage-gated K channel, in glomerular endothelium and at the apical membrane of the renal proximal tubule. J. Am. Soc. Nephrol. 2002, 13:2831-9.
- Lee SI et al.; A null mutation of mouse Kcna10 causes significant vestibular and mild hearing dysfunction. Hear. Res., 2013, 300:1-9.
- Yao X et al.; Expression of KCNA10, a voltage-gated K channel, in glomerular endothelium and at the apical membrane of the renal proximal tubule. J. Am. Soc. Nephrol.. 2002, 13 (2831-9).
Inquiry
