- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0030 | Human KCNA5 Stable Cell Line-CHO | KCNA5 | Human | Epithelial-like | INQUIRY |
| ACC-RI0131 | Human KCNA5 Stable Cell Line-HEK293 | KCNA5 | Human | Epithelial | INQUIRY |
Potassium channels are a complex type of voltage-gated ion channels. They are present in all eukaryotic cells and have multiple functions, including maintaining membrane potential, regulating cell volume, and regulating the electrical excitability of neurons. The delayed rectification function of potassium channels allows nerve cells to effectively repolarize after an action potential.
The voltage-gated potassium channels that mediate the transmembrane potassium transport in the excitable membrane are usually tetrameric potassium selective channels. Potassium ions pass through this channel according to their electrochemical gradient. And by responding to the transmembrane voltage difference, the channel alternates between open and closed conformations. It is generally composed of functional homotetramer and heterotetramer with different proportions of KCNA1, KCNA2, KCNA4, KCNA5 and possibly other family member subunits; channel properties depend on the alpha subunits that are part of the channel The type of base. The channel properties are regulated by the beta subunits, which regulate the subcellular location of the alpha subunit and promote rapid inactivation. The homotetramer channel shows rapid activation and slow inactivation.
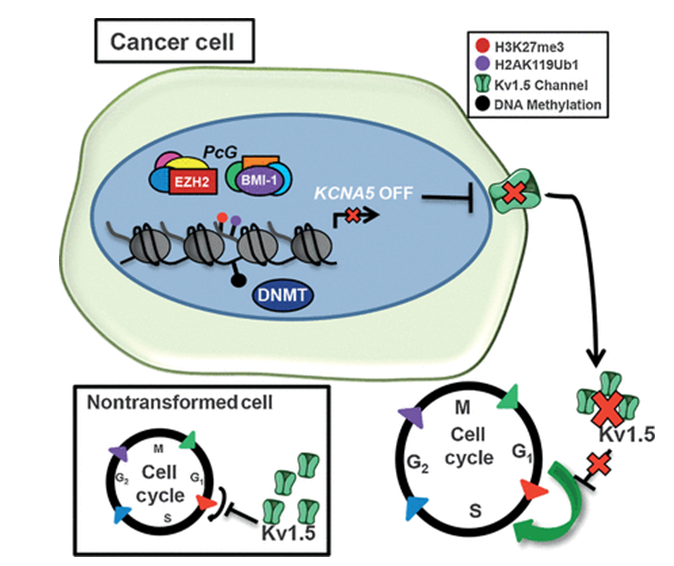
Figure.1 Overview of the epigenetic repression of KCNA5 in Ewing sarcoma cancer cells. (Katherine E. Ryland, et al.; 2016)
KCNA5 Location
The KCNA5 gene is a member of the subfamily that encodes potassium channels, voltage-gated, and oscillators. Through the study of somatic hybrids, McPherson et al. located a shaker-related potassium voltage-gated channel gene on chromosome 12. This is marked as KCNA5. After that, the researchers used multi-point linkage analysis of 8 CEPH families to locate the KCNA5 gene on chromosome 12p, and determined the relative positions of the KCNA5 gene and the 4 DNA markers.
KCNA5 Function
The cardiac repolarization process is regulated by several outward currents, among which the ultra-fast delayed rectifier potassium current (I Kur) is believed to play a major role in the repolarization of human atrial myocytes. I Kur is carried by the functional channel Kv1.5, which is assembled by the pore-forming α subunit encoded by KCNA5.
KCNA5 and Disease
Atrial fibrillation is a rhythm disorder characterized by disordered atrial electrical activity. This common disease is susceptible to stroke and heart failure, and is increasingly recognized as a genetic disease. To determine the genetic defects that confer disease susceptibility, patients with idiopathic atrial fibrillation who lack traditional risk factors were evaluated. Genomic DNA scanning revealed that a nonsense mutation in KCNA5 encodes a voltage-gated potassium channel Kv1.5 expressed in the human atrium. The loss of channel function translates into prolonged action potential and early post-depolarization of human atrial myocytes, which increases the vulnerability to triggering activities triggered by pressure. The pathogenic link between impaired Kv1.5 function and susceptibility to atrial fibrillation has been verified in a mouse model at the biological level.
The functionally defective KCNA5 is exciting as a genetic determinant of AF, especially in the optimization of AF treatment. As we all know, the existing antiarrhythmic drugs of class I or III, such as dofetilide and sotalol, may bring about ventricular repolarization, which is generally considered to be an important risk factor for fatal arrhythmia. These life-threatening side effects are due to non-selective blocking of potassium currents in atrial and ventricular cardiomyocytes, which hinder the proper application of these antiarrhythmic drugs to patients in clinical practice. Given these limitations, new drugs targeting selective currents in the atria are likely to be attractive alternatives to AF treatment, especially in the atria. I kur is the key determinant of phase I repolarization during the action potential. There is strong evidence that the expression of KCNA5 in the human atrium is much more extensive than in the ventricle, and I Kur has not been recorded in the human ventricle, implying that Kv1.5 is a potential selective target for AF medical management. Importantly, AF The traditional therapy is based on the inhibition of I Kur and is not suitable for patients with loss of Kv1.5 function. Therefore, before using Kv1.5 targeted drugs to treat AF, the patient's KCNA5 needs to be genotyped.
References
- Albrecht, B., et al.; Characterization of a voltage-activated K-channel gene cluster on human chromosome 12p13. Receptors Channels. 1995, 3: 213-220.
- Chandy, K. G., et al.; A family of three mouse potassium channel genes with intronless coding regions. Science. 1990, 247: 973-975.
- Christophersen, I. E., et al.; Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Europ. Heart J. 2013, 34: 1517-1525.
- Curran, M. E., et al.; Molecular cloning, characterization, and genomic localization of a human potassium channel gene. Genomics. 1992, 12: 729-737.
- Yang Y, et al.; Novel KCNA5 loss-of-function mutations responsible for atrial fibrillation. J Hum Genet. 2009, 54(5):277-83.
- Olson TM, et al.; Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006, 15(14): 2185-91.
- Katherine E. Ryland, et al.; Promoter Methylation Analysis Reveals That KCNA5 Ion Channel Silencing Supports Ewing Sarcoma Cell Proliferation. Mol Cancer Res. 2016, 14(1); 26-34.
Inquiry
