- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0119 | Human KCNJ3/KCNJ5 Stable Cell Line-HEK293 | KCNJ5 | Human | Epithelial | INQUIRY |
KCNJ5 gene provides instructions for the production of a potassium channel protein(G protein-activated inward rectifier potassium channel 4). This means that this gene is involved in the process of transporting positively charged potassium ions inside and outside the cell. The potassium channel composed of KCNJ5 is a G protein-sensitive internal rectifier potassium channel(Girk). Under normal physiological conditions, this channel is activated by direct interaction between the cytoplasmic N and C ends of the protein and the β subunit of the G protein. A study found that the KCNJ protein contains two transmembrane domains surrounding the P region, which contains a potassium ion selective filter. KCNJ5 can pair with KCNJ3 to form heteropolymers with high activity, or homopolymers with low to moderate activity. The potassium channels produced by the KCNJ5 gene are found in multiple tissues, including the adrenal glands, which are small hormone-secreting glands located at the top of each kidney. In these glands, the flow of ions creates a charge on the cell membrane, which affects the triggering of certain biochemical processes that regulate the production of aldosterone. Since aldosterone helps control blood pressure by maintaining proper salt and fluid levels in the body, mutations in this channel function can also cause changes in blood pressure.
KCNJ5 Cloning and Expression
In 1994, Ashford et al. cloned rat Kcnj5 and isolated the human homologue. Subsequently, the researchers discovered that the primary structure of KCNJ5 makes it belong to the internal rectifier potassium channel J subfamily, which includes KCNJ2 and KCNJ4. In 1997, scientists cloned the mouse Girk4 gene and reported a partial sequence of human Girk4. They found that Girk4 is almost exclusively expressed in mouse hearts. Further studies have found that Girk4 is significantly expressed in the mouse hypothalamus, and is most clearly expressed in the ventromedial, paraventricular, and arcuate nucleus. These neuronal populations are related to energy homeostasis. In addition, the KCNJ5 gene was also found to be significantly expressed in human skeletal muscle and heart.
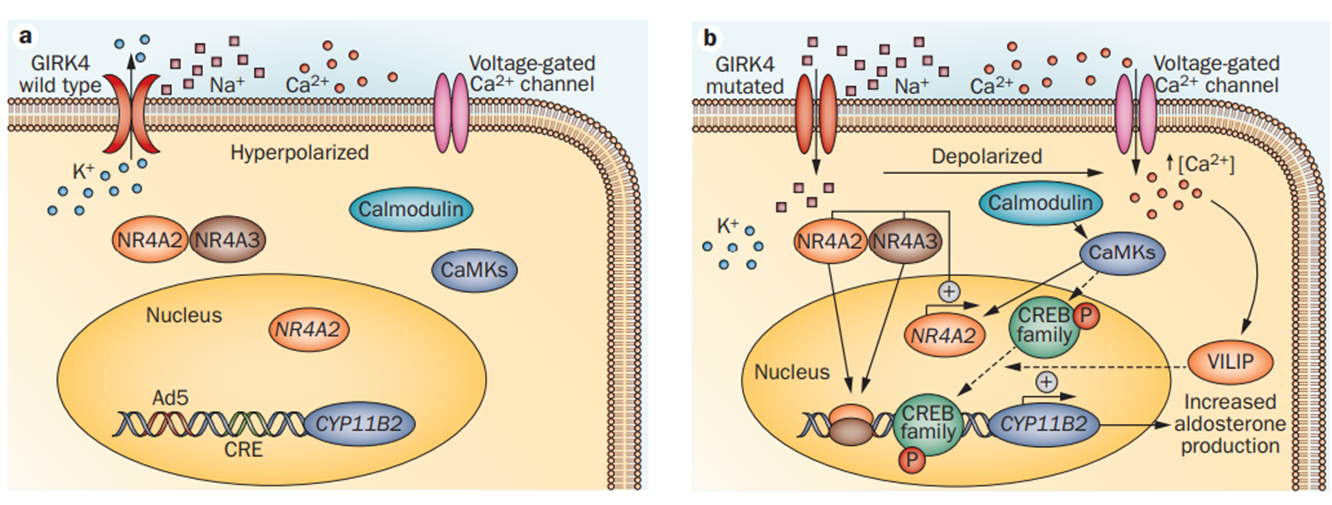
Figure 1. Effect of KCNJ5 mutations on adrenal glomerulosa cells.(Paolo Mulatero, et al.; 2013)
KCNJ5 and Disease
Aldosterone-producing Adenoma
About 40% of aldosterone adenomas are caused by mutations in the KCNJ5 gene. These adenomas are non-cancerous (benign) tumors that form in the adrenal glands. The genetic changes associated with these tumors, called somatic mutations, are acquired during a person's lifetime and only exist in the adrenal cells that produce the tumor. Mutations in the KCNJ5 gene associated with this condition change a single protein building block (amino acid) in the potassium channel. The changed potassium ion channel has lower selectivity, allowing other ions, especially sodium ions, to pass through. Sodium ions flowing into the adrenal cells affect the charge on the cell membrane, activating another channel that allows calcium ions to enter. Excessive influx of calcium ions activates the calcium/calmodulin pathway, increasing the production of aldosterone, leading to excessive aldosterone, leading to high blood pressure (hypertension) and increased risk of heart attack and stroke. Excessive activation of the calcium/calmodulin pathway in the adrenal glands also increases cell growth and division (proliferation), thereby promoting the formation of adenomas.
Familial Hyperaldosteronis
An inherited KCNJ5 gene mutation was found in patients with familial hyperaldosteronism type III. These mutations are called germline mutations and can be found in every cell of the body. Familial hyperaldosteronism causes high blood pressure, and some patients have abnormally enlarged adrenal glands (adrenal hyperplasia). As mentioned above, in aldosterone adenomas, mutations in the KCNJ5 gene result in the production of potassium channels that are less selective. The abnormal flow of ions through these channels leads to increased production of aldosterone, leading to high blood pressure.
References
- Ashford, M. L. J., et al.; Cloning and functional expression of a rat heart KATP channel. Nature. 1994, 370: 456-459.
- Perry, C. A., et al.; Predisposition to late-onset obesity in GIRK4 knockout mice. Proc. Nat. Acad. Sci. 2008, 105: 8148-8153.
- Choi M, et al.; K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011, 331(6018):768-72.
- Mulatero P, et al.; Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol. 2013, 9(2):104-12.
- Stowasser M. Primary aldosteronism and potassium channel mutations. Curr Opin Endocrinol Diabetes Obes. 2013, 20(3):170-9.
- Paolo Mulatero, et al.; Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat. Rev. Endocrinol. 2013, 9, 104-112.
Inquiry
