- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0107 | Human KCNN1 Stable Cell Line-CHO | KCNN1 | Human | Epithelial-like | INQUIRY |
Calcium-activated K+ channels (KCa) are divided into three subgroups according to their single-channel K+ conductance characteristics: large conductance (BK, KCa1.1), medium conductance (IK, KCa3.1) and small conductance (SK, KCa2.x). So far, only small conductance channels have been determined to play a role in the cell membrane of cardiomyocytes. Small conductance calcium-activated potassium SK channels are divided into three subtypes, KCa2.1(SK1), KCa2.2 (SK2) and KCa2.3(SK3), which are coded by genes KCNN1, KCNN2, and KCNN3, respectively.
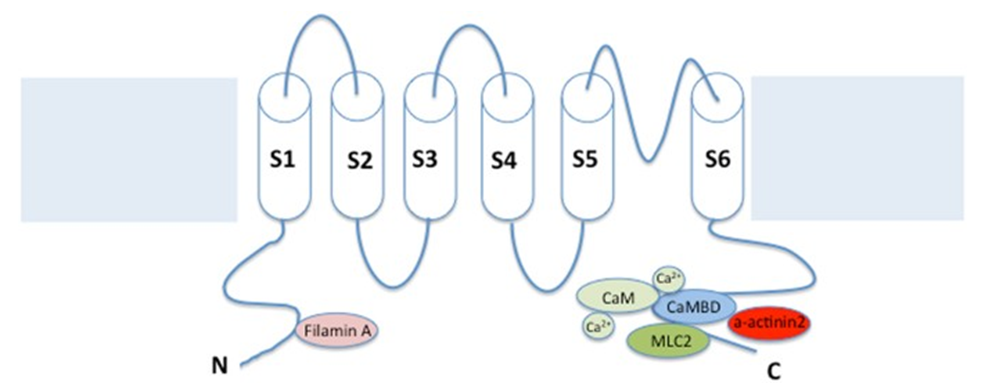
Figure 1. SK channels consist of six transmembrane regions (TMs) and a single pore loop, with four subunits around the central pore. (Gu M, et al.; 2018)
Existing structural studies have found that the channel is composed of four α subunits (KCa2.1, KCa2.2 or KCa2.3) assembled together to form a functional homotetramer or heterotetramer. SK channels are similar to other potassium channels in that they have 6 TMDs and a P-loop region, which are homologous to the pore region (between S5 and S6) of the voltage-sensitive potassium channel and the N and C ends of the cell. Unlike other potassium channels, the transmembrane segment S4 in the SK α subunit is not charged. In addition, SK channels are not co-assembled with β subunits.
KCNN1 Function
The function of KCNN1 depends on its participation in the formation of channels. Existing studies have shown that SK channels are activated by submicromolar concentrations of cytoplasmic Ca2+. Ca2+ sensitivity is attributed to the association with calmodulin, which constitutively binds to the proximal C-terminus of each α subunit and mediates gating in response to Ca2+ binding. SK channels play an important role in linking intracellular Ca2+ concentration fluctuations with membrane K+ conductance. Like vascular smooth muscle, the physiological function of SK channels in the heart may be to restrict the entry of excessive Ca2+.
The SK channel-mediated current (ISK or ISK, Ca) shows inward rectification, thereby limiting the physiologically relevant outward current branches. Similar to the K+ current of the classic inward rectifier, divalent cations such as Ca2+ or Mg2+ may explain this inward rectification.
In addition, the study also found that the SK channel subfamily all have varying degrees of sensitivity to apamin. Among them, SK1 (KCa2.1 encoded by KCNN1) has the lowest sensitivity; SK2 (KCa2.2 encoded by KCNN2) has the highest sensitivity; and SK3 (KCa2.3 encoded by KCNN3) has medium sensitivity.
In addition to the function of cardiomyocytes, the SK channel formed by KCNN1 is also involved in the process of brain neuron function. After the action potential of vertebrate neurons, post-hyperpolarization (AHP) occurs, which may last for a few seconds and may have a profound effect on the firing pattern of neurons. The increase in intracellular calcium caused by the action potential is slowly attenuated. This slow decay contributes to the persistence of hyperpolarization after the action potential is triggered.
A hierarchical analysis of the SK channel found that each component is different in kinetics and is mediated by different calcium-activated potassium channels. SK channels like KCNN1 are activated in a voltage-independent manner, and have relatively small unit conductance and high sensitivity to calcium.
Studies have found that SK channels play an important role in all excitable cells and are widely distributed throughout the brain. KCa 2.1 and KCa 2.2 subunits are expressed in the neocortex, amygdala, brainstem and hippocampus, while KCa 2.3 subunits are highly expressed in the midbrain, hypothalamus, cerebellum and thalamus. The SK channel plays an important role in neurons because it is located on the excitatory synapses and dendritic branches, spines and shafts of many central neurons. SK channels play an important role in the regulation of limbic, midbrain dopaminergic excitability and synaptic function, and cerebellar neurons, suggesting that these channels are important regulators of dopamine release in the entire brain, thus suggesting that they may be involved in Parkinson's disease.
References
- Kohler, M., et al.; Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996, 273: 1709-1714.
- V.Suppiramaniam, et al.; Comprehensive Toxicology (Second Edition). Volume 6, 2018, Pages 202-241.
- Gu M, et al.; Small-conductance Ca2+-activated K+ channels: insights into their roles in cardiovascular disease. Exp Mol Med. 2018, 50(4):1-7.
Inquiry
