- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- Nav1.8
Nav1.8
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0055 | Rat Nav1.8 Stable Cell Line-ND7-23 | Nav1.8 | Rat | Neuronal | INQUIRY |
There are 9 types of human sodium channels, Nav1.1 ~ Nav1.9. Nav1.5, Nav1.8 and Nav1.9 are tetrodotoxin (TTX) insensitive sodium channels, and Nav1.8 is involved in chronic pain, Atrial fibrillation and Budd-Chiari syndrome are important ion channels, which are highly selective targets for pain treatment. In-depth research on Nav1.8 will help clarify the pathogenesis of pain and other related diseases, develop analgesic drugs, and provide a basis for the diagnosis, treatment, and prevention of diseases.
Molecular Structure of Nav1.8
The coding gene for Nav1.8 is SCN10A, which is located in the 3p21-22 region of human chromosome and mainly encodes the α subunit. The study found that human and rat Nav1.8 gene homology is as high as 93%. The Nav1.8 molecule is composed of α and β subunits, and the α subunit is the main functional unit. Each α subunit is surrounded by 4 homology domains to form a central hole of Nav1.8. Each domain contains 6 α spiral membrane structures (S1-S6), of which the conserved S4 is sodium ion the voltage sensor of the channel. There are mainly 4 subtypes of β subunits, β1-β4, which are mainly β1 and β3 in humans. They play an auxiliary role in the positioning and stability of α in cell membranes, and also participate in the inactivation and voltage sensitivity of α subunits. The current of Nav1.8 is mainly formed by α, and the β subunit has little effect on its current.
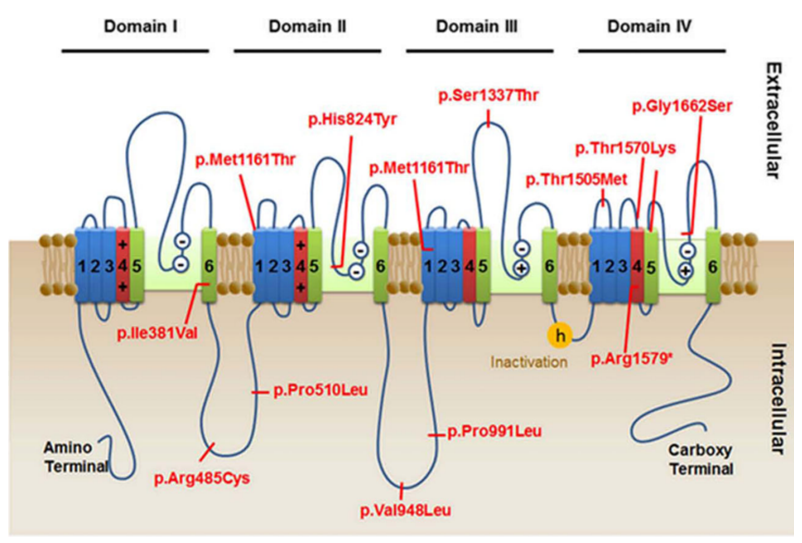
Figure 1. Location of SCN10 variants detected. (Kambouris M, et al.; 2016)
Expression of Nav1.8
The expression of Nav1.8 in rats changes with the increase of age. It starts to develop at 15 days after the embryo and reaches the adult level seven days after delivery. It is mainly restricted to the developing primary sensory neurons, and its expression level begins to decline until the old age. Studies have shown that Nav1.8 is mainly expressed in 89% C-, 93% Aδ, 60% Aα/β afferent neurons and 88% C unresponsive neurons Nav1.8 neurons, but not all Nav1.8 positive neurons are nociceptive sensory neurons. Only 20% of SA-Aβ fibers in nerve fibers are nociceptors, and Nav1.8 also plays an important role in non-noxious sensory neurons. Previous studies have suggested that Nav1.8 is mainly expressed in nociceptive neurons with medium and small diameters. However, recent data indicate that Nav1.8 is preferentially expressed in large, medium, and small diameter muscle afferent neurons, especially sensory neurons with a diameter of 20 to 70μm. Although it is now generally believed that Nav1.8 is expressed in sensory afferent nerve fibers, later studies have found that Nav1.8 can be ectopically expressed in motor nerve fibers under pathological conditions.
Nav1.8 and Disease
Due to the important role of Nav1.8 in chronic pain, people have conducted extensive research using pain models, gene knockout techniques, and specific blockers. In the chronic inflammation model, after CFA injection, the mRNA of Nav1.8 in the fork nerve increased by 2.5 times from 1 to 2 weeks. Intrathecal injection of Nav1.8 antisense nucleotides blocked the spontaneous pain and hyperalgesia caused by the SNL model and CFA. Ten days after sciatic nerve compression, the injection of Nav1.8 antisense nucleotides reduced mechanical allodynia. The persistent inflammation caused by CFA sensitized Nav1.8, and the peak current intensity of Na+ and Nav1.8 of Aβ afferent nerve fibers increased. In chronic neuropathic pain models such as SNI, SNL, and CCI, the expression of Nav1.8 in the corresponding dorsal root ganglia is up-regulated. In addition, Nav1.8 highly selective blockers, A-803467, A-887826, and aminophylline all dose-dependently blocked mechanical hyperalgesia caused by SNL, SNI, CFA and capsaicin. These studies directly indicate that Nav1.8 is involved in chronic neuropathic and chronic inflammatory pain.
Genetic analysis of patients with idiopathic small neuropathic pain found that 2/3 of Nav1.8 gene mutations increase the sodium ion depolarization response, which leads to high excitability of DRG neurons. Electrophysiological analysis of the Nav1.8/11706v mutation site of the disease found that the altered sodium channel lowered the current threshold and increased the frequency of action potentials of DRGs. Idiopathic small neuropathic pain with Nav1.8 gene mutations is more common, but patients with other dysfunctions are less common. Dabby R et al. analyzed the gene of a patient with idiopathic small neuropathic pain accompanied by gastrointestinal dysfunction and found that there is a new heterozygous mutation in the Nav1.8 gene, which may cause gastrointestinal dysfunction. These data indicate that the Nav1.8 gene mutation is one of the causes of chronic neuropathic pain and may also be one of the causes of other dysfunctions. Electrophysiological analysis found that the common mutation rs6795970 of Nav1.8 is involved in the regulation of DRG neuron excitability under non-pathological conditions, which indicates that Nav1.8 is involved in the regulation of pain sensitivity. Nav1.8 plays an important role in neuropathic and chronic inflammatory pain. It may be involved in acute and subchronic inflammatory pain, but not in acute thermal pain and acute incision pain. Nav1.8 is also a suspected cause of other diseases. At the same time, Nav1.8 also plays a role in maintaining the excitability of nociceptive sensory neurons, the firing and duration of action potentials, and the regulation of pain sensitivity. But for the role of Nav1.8 in non-noxious sensory neurons, and the role it plays in other diseases, further research is needed to reveal.
References
- Savio-Galimbertil E, et al.; SCN10A/Nav1.8 modulation of peak and late sodium currents in patients with early onset atrial fibrillation.Cardiovasc Res. 2014,104:355-363.
- Fukuyama M, et al.; Novel SCN10A variants associated with Brugada syndrome. Europace. 2016,18: 905-911.
- Dib-Hajj SD, et al.; Stucture of the sodium channel gene SCN11A:evidence for intron-to-exon conversion model and implications for gene evolution. Mol Neurobiol. 2003, 26:235-250.
- Kerr NC, et al.; Novel isoforms of the sodium channels Nav1.8 and Nav1.5 are produced by a conserved mechanism in mouse and rat. J Biol Chem. 2004, 279:24826-24833.
- Benn SC, et al.; Developmental Expression of the TTX-Resistant Voltage-Gated Sodium Channels Nav1.8 (SNS) and Nav1.9 (SNS2) in Primary Sensory Neurons. J Neurosci. 2001, 21:6077-6085.
- Wang S, et al.; Reduced thermal sensitivity and Nav1.8 and TRPV1 channelexpression in sensory neurons of aged mice. Neurobiol Aging. 2006, 27:895-903.
- Huang J, et al.; Small Fiber Neuropathy Nav1.8 Mutation Shifts Activation to Hyperpolarized Potentials and Increases Excitability of Dorsal Root Ganglion Neurons. J Neurosci. 2013, 33:14087-14097.
- Dabby R, et al.; Painful small fiber neuropathy with gastroparesis: A new phenotype with a novel mutation in the SCN10A gene. J Clin Neurosci. 2016, 26:84-88.
- Kambouris M, et al.; Biallelic SCN10A mutations in neuromuscular disease and epileptic encephalopathy. Ann Clin Transl Neurol. 2016, 4(1):26-35.
Inquiry
