- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0056 | Human TRPA1 Stable Cell Line-HEK293 | TRPA1 | Human | Epithelial | INQUIRY |
| ACC-RI0191 | Human TRPA1 Stable Cell Line-CHO | TRPA1 | Human | Epithelial-like | INQUIRY |
In 1975, Minke et al. discovered the transient receptor potential (TRP) gene in the visual conduction system of mutant Drosophila melanogaster, and the mutant Drosophila produced only transient electrophysiological signals after light stimulation, hence the name TRP. The TRP channel family is the largest ion channel family and one of the most important sensory conduction families. It exists in almost all prokaryotic and eukaryotic cells. A variety of ion channels are involved in the production of pain and respond to a variety of cold and heat,mechanical and chemical stimulation. TRP channel is a non-selective cation channel. According to amino acid sequence homology, more than 30 TRP channel family members can be divided into 7 subfamilies: TRPC, TRPV, TRPM, TRPA, TRPP, TRPML and TRPN family. It plays a key role in different homeostatic functions such as nerve regulation, body fluid regulation, acid-base balance regulation and body temperature regulation.
TRPA1 was originally thought to be a protein expressed in liposarcoma cell lines, also known as ANKTM1 protein, which is a member of the TRP family. In 1999, Jaquemar and others successfully isolated TRPA1 protein from human lung fibroblasts for the first time. TRPA1 ion channels can feel a variety of harmful stimuli, such as cold and heat, stimulating compounds, and endogenous substances related to cell damage. They can be activated by different electrophilic molecules that bind to proteins through electrostatic or covalent modification. And then, it causes pain. Therefore, it has biological effects such as mediating inflammation and pain stimulation. Clinical studies have shown that this channel is related to various diseases such as pain and itching. Blocking the TRPA1 ion channel can effectively relieve inflammatory pain and neuropathic pain. Therefore, the TRPA1 channel is a potential target for disease treatment.
Molecular Structure and Distribution of TRPA1
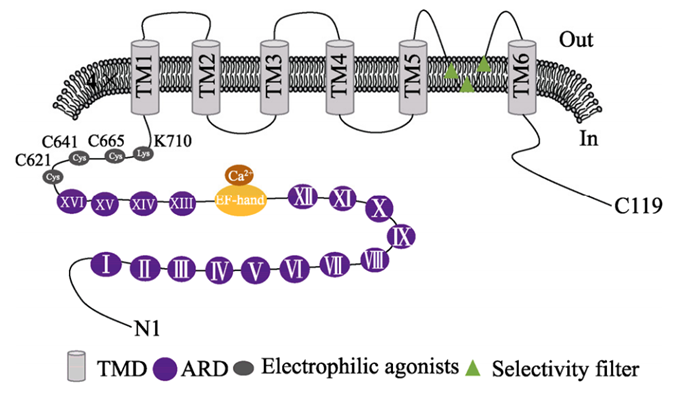
Figure 1. Schematic diagram of TRPA1 protein structure.
TRPA1 ion channel is a type of homo- or heterotetrameric non-selective cation channel located on the cell membrane or organelle membrane. The aspartic acid residue (D915) contained in the tetrameric structure has Neutralization can significantly reduce the permeability of Ca2+, but it still has a higher permeability to Ca2+ when the channel is continuously open. The human TRPA1 protein is composed of approximately 1110 amino acids and has a relative molecular mass of approximately 120-130 kDa. It is located on the human chromosome 8q13 and has 29 exons. Paulsen et al. used single-particle electron cryomicroscopy to determine the three-dimensional atomic structure of human TRPA1. Human TRPA1 is a homotetrameric structure. Each monomer has 6 transmembrane protein regions (TMD). A pore structure is formed between the 6th transmembrane domain and the hydrophilic region. This pore structure is a selective filter, in which the TM6 region is the site where the activator and the antagonist must bind. The N-terminus and C-terminus are both on the cytoplasmic side. The N-terminus occupies about 80% of the protein, while the C-terminus only occupies 14%. The C-terminal is connected to the transmembrane domain through β-sheets, TRP-like domains and open loop structures, and the positively charged domain contained in the C-terminal region can interact with negatively charged ligands; in the N-terminal structure of human TRPA1 The 16 ankyrin repeats (ARDs) present in the domain are its most significant structural feature. Each ARD is composed of 33 amino acids. The 11 ARDs at the distal end are anti-parallel helical, which can promote the coiling side chain interaction of the C-terminal region and participate in the channel transport of the plasma membrane. Due to the existence of a large number of ankyrin repeats, the protein has strong foldability, which can maintain the structure of the protein and play an important role in the gating mechanism of the TRPA1 channel. The N-terminal EF-hand domain is the most common site where Ca2+ interacts with proteins. Electron microscope structure simulation predictions show that there are a large number of cysteine residues at the N-terminus of TRPA1 protein that can form a binding pocket for ligands, which can undergo a covalent addition reaction with electrophilic activators, leading to conformational changes and opening of channels. Lysine and cysteine residues located between the ankyrin and TM1 regions are the key sites for electrophilic and reactive agonists to activate the TRPA1 channel.
Distribution of TRPA1 Ion Channels
TRPA1 protein has been found in many species including humans, and homologous genes have been identified and cloned in rodents, dogs, cows, pigs and other mammals, birds, fish, amphibians, insects and nematodes. It is distributed in various organs such as lung, heart, pancreatic islets and gastrointestinal tract, and is widely expressed in sensory neurons and non-nerve cells. Studies believe that TRPA1 protein is co-expressed with TRPV1 protein in most cases, and TRPA1 protein is mainly expressed in a large number of primary sensory neuron subgroups, including dorsal root ganglia, trigeminal ganglia, vagus ganglia and nodular ganglia. In the central nervous system, TRPA1 is expressed in hippocampal neurons, astrocytes, and oligodendrocytes. In addition, studies have found that the inner ear fiber cells of mice also express TRPA1 and participate in auditory conduction. It is abundantly expressed in the peripheral nervous system in rat, such as, pancreatic cells, vascular endothelial cells, enterochromaffin cells, human breast cells, keratinocytes, melanocytes, cardiopulmonary fibroblasts, prostate epithelial cells, dental pulp fibroblasts, cardiac muscle cells, gastrointestinal mucosa, brain, kidney epithelial cells, hepatocytes, T lymphocytes, and B lymphocytes.
Biological Characteristics of TRPA1 Ion Channels
In mammals, TRPA1 is highly expressed in the terminals of small and medium diameter primary sensory neurons, and it relies on nociceptors to sense harmful stimuli in the environment. Studies have shown that TRPA1 can be sensitive to low temperature, mechanical and chemical stimuli, leading to calcium and sodium ions influx, depolarization of cell membranes, causing the generation of neuronal action potentials, and ultimately causing pain and inflammation, while blocking TRPA1 ions The channel can effectively reduce the hyperalgesia and allodynia induced by noxious stimuli. Therefore, the TRPA1 ion channel is of great significance for maintaining the normal physiological functions of the body.
Ca2+-mediated Regulation of TRPA1
Intracellular and extracellular Ca2+ is a key regulator of TRP channels and an important ion for activating and regulating the activity of TRPA1 channels. The TRPA1 structure contains the EF-hand helical domain that can bind to Ca2+. This domain is the most common binding site for protein interaction with Ca2+, and the three amino acid residues of this domain are related to the activation of Ca2+-dependent channels. Studies have reported that increased intracellular and extracellular Ca2+ levels can activate TRPA1 and enhance the activity of this channel under chemical stimulation. However, Ca2+ influx can also quickly inactivate the channel. When the local cell Ca2+ concentration is too high, it may cause rapid inactivation of the channel without extracellular Ca2+. In the case of, the activation and deactivation of the channel are both delayed. Although the calcium-dependent regulation of TRPA1 is essential, its underlying mechanism remains unclear. In recent years, studies have found that the Ca2+ regulation of TRPA1 depends on calmodulin (CaM), and CaM combines with TRPA1 to form a calcium-sensitive channel complex. As a Ca2+ sensor and effector, CaM regulates the sensitivity of TRPA1 in resting and activated states through the interaction of the carboxyl group of CaM and the C-terminal calmodulin binding domain of TRPA1. Therefore, Ca2+ is one of the endogenous regulators of TRPA1, which can rapidly activate the channel after increasing the intracellular and extracellular Ca2+ concentration and enhance the response to other agonists, but the mechanism of its action is still controversial.
TRPA1 Ion Channel can be used as a Mechanically Sensitive Channel
TRPA1 can sense various forms of mechanical stimulation inside and outside the body, and also plays an important role in cell signal transduction. Because a large number of ankyrin repeats at the N-terminus of the TRPA1 protein can form a connection between the cellular protein skeleton and mechanical stimulation, it can regulate the opening of the TRPA1 ion channel. Studies have found that TRPA1 is expressed in astrocytes in the nerve endings of the spinal cord. Mechanical stimulation of the dorsal spinal cord neurons in mice causes nerve damage and pain; puerarin can block the TRPA1 and Increased expression of TRPV1 mRNA; after the rat dorsal root ganglion was treated with hypertonic fluid, a single-channel current was recorded by the patch clamp, and this current could be blocked by the TRPA1 antagonist camphor, indicating that TRPA1 may be involved in the osmotic pressure Transduction of mechanical stimuli caused by changes; mechanical hyperalgesia after burns can be blocked by the antagonist HC-030031; mice were injected with Freund's complete adjuvant (CFA) to induce mechanical hyperalgesia, and TRPA1 antagonist was given After AP18, the above-mentioned pain symptoms were significantly relieved. In summary, noxious mechanical stimulation can activate TRPA1 ion channels, but can be blocked by TRPA1 antagonists.
References
- MINKE B, et al.; Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975, 258(5530): 84-87.
- SMANI T, et al.; Functional and physiopathological implications of TRP channels. Biochim Biophys Acta. 2015, 1853(8): 1772-1782.
- CLAPHAM D E, et al.; International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: Transient receptor potential channels. Pharmacol Rev, 2003, 55(4): 591-596.
- LINDSAY C D, TIMPERLEY C M. TRPA1 and issues relating to animal model selection for extrapolating toxicity data to humans. Hum Exp Toxicol. 2020, 39(1): 14-36.
- NILIUS B, et al.; The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflügers Arch-Eur J Physiol, 2012, 464(5): 425-458.
- HAMILTON N B, et al.; Proton-gated Ca(2+)- permeable TRP channels damage myelin in conditions mimicking ischaemia[J]. Nature, 2016, 529(7587): 523-527.
- KURGANOV E, et al.; Requirement of extracellular Ca2+ binding to specific amino acids for heat-evoked activation of TRPA1. J Physiol, 2017, 595(8): 2451-2463.
- ASGAR J, et al.; The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience, 2015(310): 206-215.
Inquiry
