- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- hcn4
hcn4
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0014 | Human HCN4 Stable Cell Line-CHO | HCN4 | Human | Epithelial-like | INQUIRY |
| ACC-RI0102 | Human HCN4 Stable Cell Line-HEK293 | HCN4 | Human | Epithelial | INQUIRY |
The HCN4 gene provides instructions to make a positively charged atom (ion) into the heart muscle cell channel. This channel is mainly located in the sinoatrial node (SA), which is a special cell area in the heart that acts as a natural pacemaker. The HCN4 channel allows potassium and sodium ions to flow into the sinoatrial node cells. This ion current is often referred to as "pacemaker current" because the electrical pulses it generates start every heartbeat and participate in maintaining a regular heart rhythm.
Electrophysiological studies on the three expressed HCN4 channels show that the activation voltage of this channel depends on membrane hyperpolarization and has unique activation kinetics. The activation of HCN channels is directly regulated by cyclic nucleotides. However, the regulatory effect depends on the subtype. Compared with HCN1 channel, cAMP can promote the activation of HCN2 and HCN4. The regulation of cAMP is mediated by the direct binding of cAMP to the cyclic nucleotide binding domain (CNBD) at the C-terminus of the cell, thereby releasing the inhibitory effect of the C-terminus on channel activation. In addition, studies have found that HCN4 is mainly expressed in some areas of the thalamic nucleus, olfactory bulb, and heart.
The HCN4 channel is responsible for hyperpolarization to activate funny current (If), which is essential for the autonomy of the sinus node. This can be seen in transgenic mice whose heart specifically knocks out the HCN4 gene, resulting in severe progressive bradycardia. These channels are highly expressed in the sinus node, and mutations in the HCN4 gene have also been found to be associated with familial sinus syndrome. These loss-of-function mutations lead to the reduction of If in the range of diastolic depolarization of sinoatrial node cells, which leads to impaired pulse generation and different phenotypes.
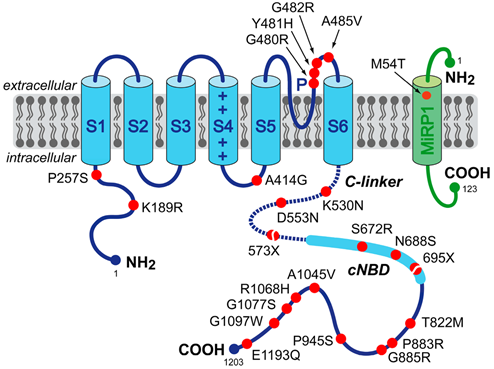
Figure 1.Schematic topology of the HCN4 and MiRP1 proteins. (Arie O. Verkerk, et al.; 2015)
HCN4 and Disease
Sinus Syndrome (SND)
At least five mutations in the HCN4 gene have been found in people with sick sinus syndrome, a heart disease that affects the function of SA nodes. So far, five variants have been found in different families: 1. Sinus bradycardia, chronopathic insufficiency and atrial fibrillation caused by the deletion of a single base pair in the fifth exon of the HCN4 gene; 2. HCN4 c -Sinus node dysfunction, QT prolongation and polymorphic ventricular tachycardia caused by missense mutations of the linker; 3. Sinus bradycardia caused by mutations in HCN4 near the cyclic AMP binding site; 4. Located Point mutations in the HCN4 ion channel pores cause sinus bradycardia; 5. Mutations in the HCN4 channel pores cause sinus bradycardia. Most of these mutations change a single protein building block (amino acid) in the HCN4 channel. In other cases, the channel is in the correct position, but the structure is abnormal, which can change the way the ions flow through. All mutations reduce the overall flow of ions into the SA node cells, thereby preventing them from generating electrical signals that control the heartbeat. These changes increase the risk of abnormal heartbeat (bradycardia), which can cause dizziness, fainting (syncope) and related symptoms. Mutations in the HCN4 gene are also found in people who have a slow heart rhythm without any other symptoms (asymptomatic bradycardia).
In addition to sinus syndrome, studies have found that HCN4 gene has a certain relationship with Brugada syndrome and left ventricular intact disease.
References
- Baruscotti M, et al.; HCN-related channelopathies. Pflugers Arch. 2010, 460(2):405-15.
- Milanesi R, et al.; Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006, 354(2):151-7.
- Netter MF, et al.; The HCN4 channel mutation D553N associated with bradycardia has a C-linker mediated gating defect. Cell Physiol Biochem. 2012, 30(5):1227-40.
- Nof E, et al.; Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation. 2007, 116(5):463-70.
- Arie O. Verkerk, et al.; Pacemaker Activity of the Human Sinoatrial Node: An Update on the Effects of Mutations in HCN4 on the Hyperpolarization-Activated Current. Int. J. Mol. Sci. 2015, 16(2), 3071-3094.
Inquiry
