- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
Gamma-aminobutyric acid (GABA) receptors are a family of protein receptors involved in GABA neurotransmission in the mammalian central nervous system. GABRG2 is a member of the GABA-A receptor gene family of heteropentameric ligand-gated ion channels. It is mainly involved in the passage of the inhibitory neurotransmitter GABA in the mammalian brain. GABA-A receptors are the target sites of many important pharmacological effects, including barbiturates, benzodiazepines and ethanol.
GABRG2 Cloning and Expression
Pritchett et al. cloned a cDNA encoding a new GABA-A receptor subunit in 1989. They called it gamma-2 (GABRG2), which shares approximately 40% sequence identity with α and β subunits. Through deduction and analysis, it is found that 468 amino acids constitute the signal sequence. The β structure loops connected by disulfide bonds have 3 potential N-glycosylation sites at the extracellular N-terminus and 4 transmembrane fragments at the C-terminus. The false site of tyrosine phosphorylation is located between transmembrane domains 3 and 4. Studies have found that GABRG2 mRNA is significantly located in neuronal subgroups throughout the central nervous system. Jin et al. identified the third splice variant of human γ-2, which they called γ-2XL. This spliceosome inserts another 40 amino acids in the long N-terminal extracellular domain. Quantitative PCR analysis detected the expression of γ-2XL and γ-2S in the whole brain regions of adults and fetuses and found that the expression of γ-2S was dominant in all tissues.
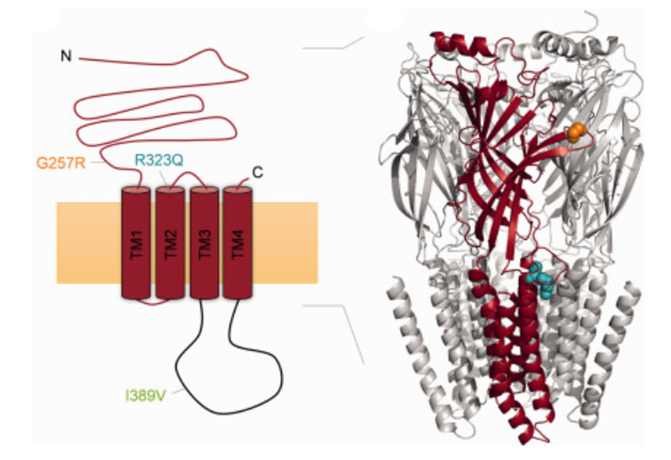
Figure 1. Schematic representation of GABAA-R γ2 subunit and Crystal structure of the ß subunit. (Eva Maria Reinthaler, et al.; 2015)
GABRG2 Gene Function
The researchers found through the whole-cell patch clamp technique that all three human GABA subunits (that is, α, β, and gamma-2) are co-expressed to promote large GABA inwardly induced currents. Because this process requires all three subunits to form high-affinity binding sites for benzodiazepines. The GABA-A ligand-gated channel selectively binds to the dopamine D5 receptor through the direct binding of the D5 carboxyl terminal domain to the second intracellular loop of the GABA-A gamma-2 receptor subunit. This physical association makes these receptor systems have the function of mutual inhibition. In addition, zinc ions regulate GABA-A receptors by inhibiting receptor function through an allosteric mechanism, which mainly depends on the composition of receptor subunits.
GABRG2 and Disease
Familial Febrile Convulsion
Existing studies have found that the destruction of GABAergic neurotransmission mediated by γ-aminobutyric acid is related to epilepsy. Researchers conducted statistics among 13 members of a French family of 3 generations and found that their epileptic seizure phenotype was most consistent with familial febrile convulsions. Further research found that the GABRG2 gene has a heterozygous missense mutation (K289M) in the patient's body.
Later, scientists discovered another heterozygous missense mutation (R43Q) in the GABRG2 gene in an affected member of a 4-generation Australian family with FEB8. And some of these mutation carriers also suffer from childhood absence seizures (ECA2). Therefore, researchers believe that hyperfebrile epilepsy (FS) and childhood absence epilepsy (CAE) have different ages of onset, and their physiology is also different. Among them, absence and seizures are obvious. This indicates that the mutation is age-dependent on different neuronal networks, which in turn affects the expression of these clinically different but genetically related epileptic phenotypes.
Developmental and Epileptic Encephalopathy
Six different de novo heterozygous missense mutations in GABRG2 were identified in 8 children unrelated to developmental and epileptic encephalopathy by Shen et al. Mutations occur in different domains of the entire gene. Therefore, in vitro functional expression studies showed different effects: these variants reduced GABA-induced currents to varying degrees and altered zinc sensitivity. Some mutations lead to reduced effectiveness of GABA stimulation, while other mutations lead to abnormal channel dynamics. The mutant protein is stable in transfected HEK293 cells, but shows different surface and intracellular expression, which indicates that some mutants have impaired transport and abnormal retention in the endoplasmic reticulum.
References
- Audenaert, D., et al.; A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006, 67: 687-690.
- Baulac, S., et al.; First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma-2-subunit gene. Nature Genet. 2001, 28: 46-48.
- Buckwalter, M. S., et al.; Localization of the human chromosome 5q genes Gabra-1, Gabrg-2, I1-4, I1-5, and Irf-1 on mouse chromosome 11. Mammalian Genome. 1992, 3: 604-607.
- Chaumont, S., et al.; Agonist-dependent endocytosis of gamma-aminobutyric acid type A (GABA-A) receptors revealed by a gamma-2(R43Q) epilepsy mutation. J. Biol. Chem. 2013, 288: 28254-28265.
- Eva Maria Reinthaler, et al.; Rare variants in GABAA receptor genes in Rolandic epilepsy and related syndromes. Annals of Neurology. 2015, 77(6).
Inquiry
