- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0051 | Human SCN5A Stable Cell Line-HEK293 | SCN5A | Human | Epithelial | INQUIRY |
| ACC-RI0164 | Human SCN5A Stable Cell Line-CHO | SCN5A | Human | Epithelial-like | INQUIRY |
The SCN5A gene belongs to a gene family that provides instructions for making sodium channel components. Under normal physiological conditions, these channels open and close at specific times to control the flow of positively charged sodium ions into the cell. Further research found that sodium channels containing proteins produced by the SCN5A gene are abundant in heart muscle cells and play a key role in the ability of these cells to generate and transmit electrical signals. These channels play an important role in the signal for the start of each heartbeat, coordinating the contraction of the upper and lower chambers of the heart, and maintaining a normal heart rate.
SCN5A Cloning and Expression
Gellens et al. cloned and identified the cardiac sodium channel gene SCN5A. After further research, the researchers found that the structure of the protein with a length of 2016 amino acids is similar to the previous sodium channel structural protein. It contains 4 homologous domains, and each homologous domain has 6 putative transmembrane regions. Sequence analysis revealed that SCN5A consists of 28 exons, spanning approximately 80 kb.
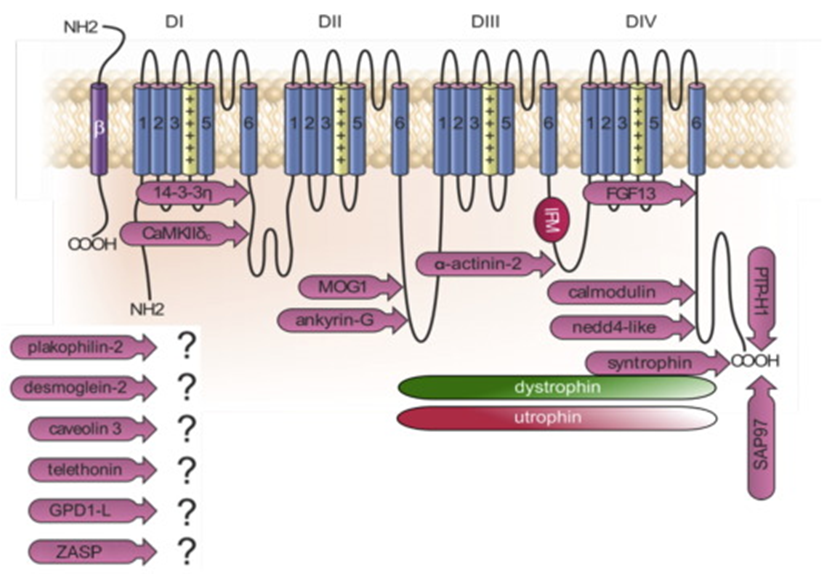
Figure 1. Topology of NaV1.5 and its interaction with various regulatory proteins. (Zaklyazminskaya E, et al.; 2016)
Subsequent studies found that the fetal and adult subtypes of SCN5A produced by alternative splicing differ in the inclusion sequences of alternative exons 6a and 6b, respectively. Among them, the two exons No. 6 both contain a 92 bp sequence, which encodes 7 different amino acids in the voltage sensor region of SCN5A domain I.
SCN5A and Disease
Brugada syndrome is a heart disease characterized by arrhythmia. In patients with Brugada syndrome, more than 400 SCN5A gene mutations have been found. Mutations in the SCN5A gene can also cause Sudden Night Death Syndrome (SUNDS), a disease first seen in people in Southeast Asia. Researchers later determined that SUNDS and Brugada syndrome are the same disease. Studies have found that some mutations in the SCN5A gene associated with Brugada syndrome change a single amino acid in the SCN5A protein. These mutations changed the structure of the ion channel formed by the SCN5A protein and disrupted the flow of sodium ions into the cardiomyocytes. In addition, other mutations also prevent the SCN5A gene from producing any functional ion channels, which also reduces the influx of sodium ions. The interruption of ion transport changes the way the heart beats, leading to arrhythmias often found in Brugada syndrome and SUNDS.
Progressive Familial Heart Block
Clinical studies have found that some mutations in the SCN5A gene have been found to cause progressive familial heart block. This condition changes the normal beating of the heart and may cause fainting (syncope) or cardiac arrest and death. Mutations in the SCN5A gene change a single amino acid in the SCN5A protein. The channel made of this mutated protein allows only a small amount or does not allow sodium ions to enter the cell. It is difficult for cardiomyocytes with these altered channels to produce and transmit electrical signals that coordinate the normal heartbeat. This interruption of the signal is called a heart block. The damaged cardiomyocytes die, leading to the accumulation of scar tissue (fibrosis) over time, thereby worsening the heart block.
At least 238 SCN5A gene mutations are currently known to cause Romano-Ward syndrome, which is the most common form of arrhythmia known as long QT syndrome. Mutations in this gene account for 5% to 10% of Romano-Ward syndrome cases. In people with this disease, the heart muscle takes longer than usual to recharge between beats. The SCN5A gene mutations that cause Romano-Ward syndrome include the change of a single amino acid and the deletion or insertion of a small number of amino acids in the SCN5A protein. The channels formed by these altered SCN5A proteins are open longer than normal, which allows sodium ions to continue to flow abnormally into cardiomyocytes. The delay in channel closure changes the transmission of electrical signals from the heart, increasing the risk of irregular heartbeats leading to syncope or sudden death.
Sick Sinus Syndrome
At least 16 mutations in the SCN5A gene have been found to cause another type of heart disease called sick sinus syndrome. This condition will affect the function of the sinoatrial node, which is a special cell area in the heart that functions as a natural pacemaker. Mutations in the SCN5A gene that cause sick sinus syndrome result in the production of non-functional sodium channels or abnormal channels that cannot transport ions normally. The flow of these ions is the key to generating electrical impulses, which initiate every heartbeat and propagate these signals to other parts of the heart. The mutation reduces the flow of sodium ions, thereby changing the ability of the sinus node to generate and transmit electrical signals. These changes increase the risk of abnormally fast or slow heartbeats, which can lead to dizziness, dizziness, fainting, and related symptoms.
Other Disorders
Variations in the SCN5A gene are associated with several other heart diseases. These include potentially life-threatening forms of arrhythmia called atrial fibrillation and ventricular fibrillation. Gene mutations associated with these diseases can alter the flow of sodium ions in the channels, leading to arrhythmia and affecting the heart's ability to supply blood. SCN5A gene mutations have also been found in some cases of sudden infant death syndrome (SIDS). Sudden Infant Death Syndrome is the leading cause of death among infants under one year old. It is characterized by sudden and unexplainable death, usually during sleep. Researchers are working to determine how changes in the SCN5A gene cause SIDS. Other genetic and environmental factors, many of which have not been identified, also play a role in determining the risk of this disease. Certain medications, including those used to treat arrhythmias, infections, epilepsy, and psychosis, may cause abnormal heart rhythms in some people. The heart disease caused by this drug, known as Acquired Long QT Syndrome, increases the risk of cardiac arrest and sudden death. A small number of acquired long QT syndromes occur in people with potentially altered SCN5A genes.
References
- Brugada P. Brugada syndrome: More than 20 years of scientific excitement. J Cardiol. 2016, 67(3):215-20.
- Butters TD, et al.; Mechanistic links between Na+ channel (SCN5A) mutations and impaired cardiac pacemaking in sick sinus syndrome. Circ Res. 2010, 107(1):126-37.
- Gui J, et al.; Multiple loss-of-function mechanisms contribute to SCN5A-related familial sick sinus syndrome. PLoS One. 2010, 5(6):e10985.
- Herfst LJ, et al.; Trafficking and functional expression of cardiac Na+ channels. J Mol Cell Cardiol. 2004, 36(2):185-93.
- Juang JM, et al.; Brugada syndrome-an under-recognized electrical disease in patients with sudden cardiac death. Cardiology. 2004;101(4):157-69.
- Shimizu W, et al.; Mechanisms of disease: current understanding and future challenges in Brugada syndrome. Nat Clin Pract Cardiovasc Med. 2005, 2(8):408-14.
- Zaklyazminskaya E, Dzemeshkevich S. The role of mutations in the SCN5A gene in cardiomyopathies. Biochim Biophys Acta. 2016, 1863(7 Pt B):1799-805.
Inquiry
