- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0053 | Human SCN9A Stable Cell Line-HEK293 | SCN9A | Human | Epithelial | INQUIRY |
| ACC-RI0170 | Human SCN9A Stable Cell Line-CHO | SCN9A | Human | Epithelial-like | INQUIRY |
The SCN9A gene belongs to a gene family, which encodes a protein that is a component of sodium channels. These sodium channels transport positively charged sodium ions into the cell and play a key role in the cell's ability to generate and transmit electrical signals. Among them, the SCN9A gene encodes the α subunit of the sodium channel of NaV1.7. Existing studies have found that NaV1.7 sodium channels exist in nerve cells called pain receptors, whose main function is to transmit pain signals. Pain receptors are part of the peripheral nervous system. It connects the brain and spinal cord with cells that detect touch, smell, and pain. The center of the pain receptors is called the cell body and is located in the dorsal root ganglia of the spinal cord. Among them, the cell body is called axon, and the fiber extends from the cell body to the whole body to receive sensory information. The axon transmits information back to the dorsal root ganglion, and the dorsal root ganglion transmits the information to the brain. In addition, NaV1.7 sodium channels are also present in olfactory sensory neurons that are responsible for transmitting olfactory-related signals to the brain.
SCN9A Cloning and Expression
The SCN9A research began with a voltage gate-controlled sodium channel cloned from a cDNA library of human medullary thyroid cancer cells, which the scientists named NENA. Sequence analysis showed that the gene encodes a 1,977 amino acid polypeptide (Nav1.7), which consists of 4 domains, each of which contains 6 transmembrane domains and 2 highly conserved pore-forming fragments. This sequence is highly similar to voltage-gated sodium channels in the brain and skeletal muscle. After further research, it was found that NENA was expressed in human c-cell cancer cell lines, rat dorsal root ganglion, adrenal gland and thyroid tissue, and smooth muscle cells cultured in human bronchus, pulmonary artery and large coronary artery.
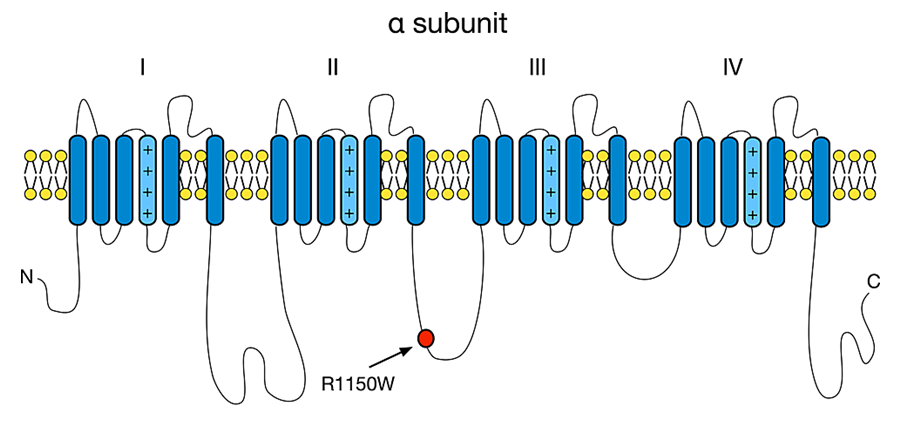
Figure 1. Nav1.7 alpha-subunit structure. (Janicki PK, et al.; 2019)
SCN9A and Disease
Congenital Insensitivity to Pain
Studies have found that at least 13 mutations in the SCN9A gene have been found to cause congenital pain insensitivity. This mutation inhibits the ability to perceive physical pain. Studies have found that mutations in the SCN9A gene that cause insensitivity to congenital pain can generate a premature stop signal in the alpha subunit generation command of the NaV1.7 sodium channel. Therefore, a shortened, non-functional subunit is produced that cannot be incorporated into the channel, resulting in the loss of the functional NaV1.7 sodium channel. The lack of these channels will hinder the transmission of pain signals from the injured site to the brain, causing patients to be insensitive to pain. The loss of this channel of olfactory sensory neurons may impair the transmission of olfactory-related signals to the brain, resulting in complete loss of smell.
Researchers have found that more than 10 mutations in the SCN9A gene can cause erythematous limb pain. Erythematous limb pain is a symptom of pain, redness and swelling in various parts of the body, especially the hands and feet. All the identified mutations changed the amino acid of the protein in the NaV1.7 sodium channel. These mutations cause the NaV1.7 sodium channel to be opened more easily than usual, and the opening time is longer than usual, which increases the flow of sodium ions, thereby generating nerve impulses in the pain receptors. The increase of sodium ions enhances the transmission of pain signals, leading to the signs and symptoms of erythematous limb pain.
Paroxysmal Extreme Pain Disorder
Approximately 10 of SCN9A gene mutations have been found to cause paroxysmal extreme pain disorders. This condition is characterized by episodes of severe pain, accompanied by redness and warmth of the skin, and sometimes convulsions and changes in breathing and heart rate. The mutation that caused this changed a single amino acid in the alpha subunit of the NaV1.7 sodium channel. Therefore, when the sodium channel is closed, it is not completely closed, causing the sodium ions to flow abnormally to the pain receptors. The increase of sodium ions enhances the transmission of pain signals, leading to pain episodes in patients with paroxysmal extreme pain disorders.
Small Fiber Neuropathy
Mutations in the SCN9A gene account for approximately 30% of cases of small fiber neuropathy. This disease is characterized by severe pain episodes and decreased ability to distinguish between hot and cold. The mutation that caused this changed a single amino acid in the alpha subunit of the NaV1.7 sodium channel. Due to the change of the α subunit, the sodium channel is not completely closed when it is closed, causing the sodium ions to flow abnormally to the pain receptors. The increase of sodium ions enhances the transmission of pain signals. In this case, the small fibers (axons) that extend from the pain receptors and transmit pain signals will degenerate over time. The reason for this degradation is not clear, but it may explain signs and symptoms such as loss of temperature differences.
Other Disorders
In a group of people affected by febrile epilepsy, at least three mutations in the SCN9A gene were found. Febrile epilepsy is a seizure caused by a high fever. Febrile epilepsy is the most common type of epilepsy in young children, affecting 2% to 5% of children in Europe and North America. Children with febrile epilepsy have a 2% to 9% chance of non-febrile epilepsy in later life. The mutations that caused these conditions changed a single amino acid in the alpha subunit of the NaV1.7 sodium channel. It is still unclear how changes in sodium channels cause febrile seizures.
References
- Cox JJ, et al.; An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006, 444(7121):894-8.
- Dib-Hajj SD, et al.; From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007, 30(11):555-63.
- Dib-Hajj SD, et al.; Genetics and molecular pathophysiology of Na(v)1.7-related pain syndromes. Adv Genet. 2008, 63:85-110.
- Doty CN. SCN9A: another sodium channel excited to play a role in human epilepsies. Clin Genet. 2010, 77(4):326-8.
- Drenth JP, et al.; Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 2007, 117(12):3603-9.
- Faber CG, et al.; Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012, 71(1):26-39.
- Janicki PK, et al.; Sporadic Erythromelalgia Associated with a Homozygous Carrier of Common Missense Polymorphism in SCN9A Gene Coding for NaV1.7 Voltage-gated Sodium Channel. Cureus. 2019, 11(5):e4587.
Inquiry
